1. Todd KH, Ducharme J, Choiniere M, Crandall CS, Fosnocht DE, Homel P, et al. Pain in the emergency department: results of the pain and emergency medicine initiative (PEMI) multicenter study. J Pain. 2007; 8:460–466. PMID:
17306626.

2. Merskey H. Classification of chronic pain: description of chronic pain syndromes and definition of pain terms. Pain. 1986; (Suppl 3):S1–S225. PMID:
3461421.
3. McAloon C, O'Connor PC, Boyer M. Patient's perception of pain on admission and discharge from the emergency department. N J Nurse. 2003; 33:7. PMID:
14692389.
4. Closs SJ, Gardiner E, Briggs M. Outcomes of a nursing intervention to improve postoperative pain at night. Acute Pain: Int J Acute Pain Manag. 1998; 1:22–31.
5. Briggs M, Closs JS. A descriptive study of the use of visual analogue scales and verbal rating scales for the assessment of postoperative pain in orthopedic patients. J Pain Symptom Manage. 1999; 18:438–446. PMID:
10641470.

6. Breivik H, Borchgrevink PC, Allen SM, Rosseland LA, Romundstad L, Breivik Hals EK, et al. Assessment of pain. Br J Anaesth. 2008; 101:17–24. PMID:
18487245.

7. Li L, Liu X, Herr K. Postoperative pain intensity assessment: a comparison of four scales in Chinese adults. Pain Med. 2007; 8:223–234. PMID:
17371409.

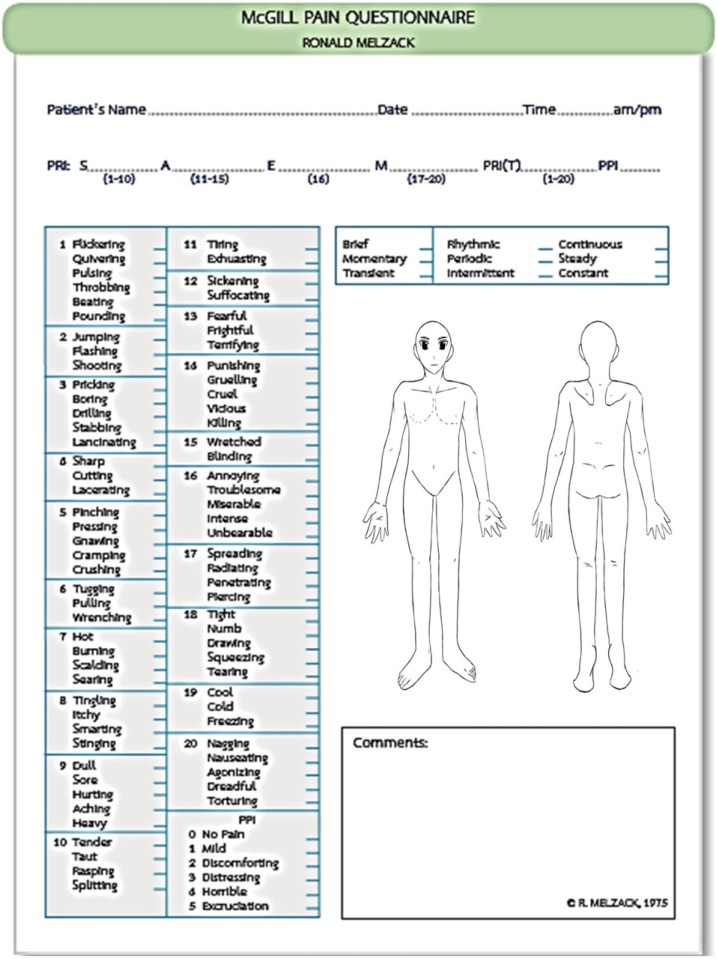
8. Melzack R. The McGill Pain Questionnaire. Anesthesiology. 2005; 103:199–202. PMID:
15983473.

9. Flaherty SA. Pain measurement tools for clinical practice and research. AANA J. 1996; 64:133–140. PMID:
9095685.
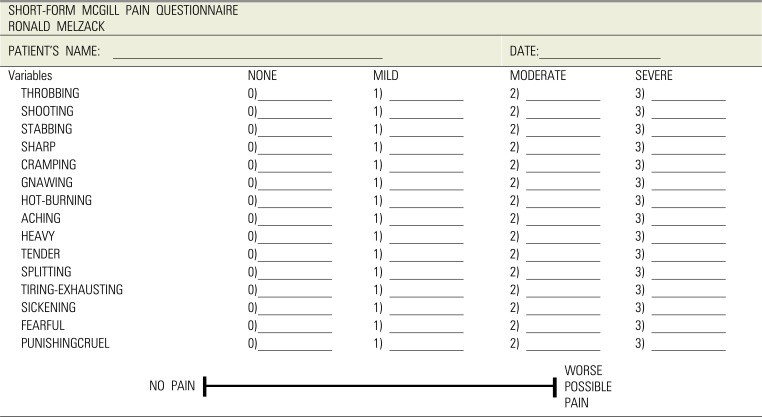
10. Melzack R. The short-form McGill Pain Questionnaire. Pain. 1987; 30:191–197. PMID:
3670870.

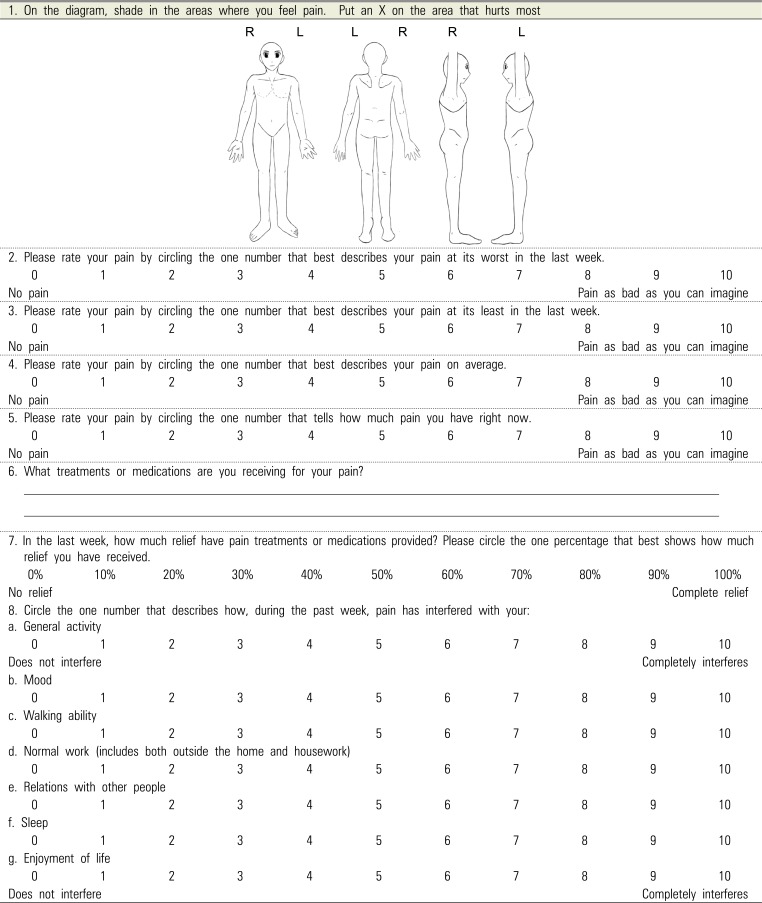
11. Daut RL, Cleeland CS, Flanery RC. Development of the Wisconsin Brief Pain Questionnaire to assess pain in cancer and other diseases. Pain. 1983; 17:197–210. PMID:
6646795.

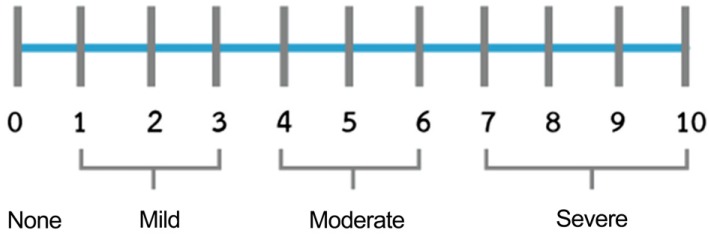
12. Heft MW, Parker SR. An experimental basis for revising the graphic rating scale for pain. Pain. 1984; 19:153–161. PMID:
6462727.

13. Keele KD. The pain chart. Lancet. 1948; 2:6–8. PMID:
18871952.

14. Downie WW, Leatham PA, Rhind VM, Wright V, Branco JA, Anderson JA. Studies with pain rating scales. Ann Rheum Dis. 1978; 37:378–381. PMID:
686873.

15. Bieri D, Reeve RA, Champion GD, Addicoat L, Ziegler JB. The Faces Pain Scale for the self-assessment of the severity of pain experienced by children: Development, initial validation, and preliminary investigation for ratio scale properties. Pain. 1990; 41:139–150. PMID:
2367140.

16. Cote CJ, Lerman J, Anderson B. A practice of anesthesia for infants and children. 5th ed. Elsevier Health Sciences: 2013;940.
17. Garra G, Singer AJ, Taira BR, Chohan J, Cardoz H, Chisena E, et al. Validation of the Wong-Baker FACES Pain Rating Scale in Pediatric Emergency Department Patients. Acad Emerg Med. 2010; 17:50–54. PMID:
20003121.

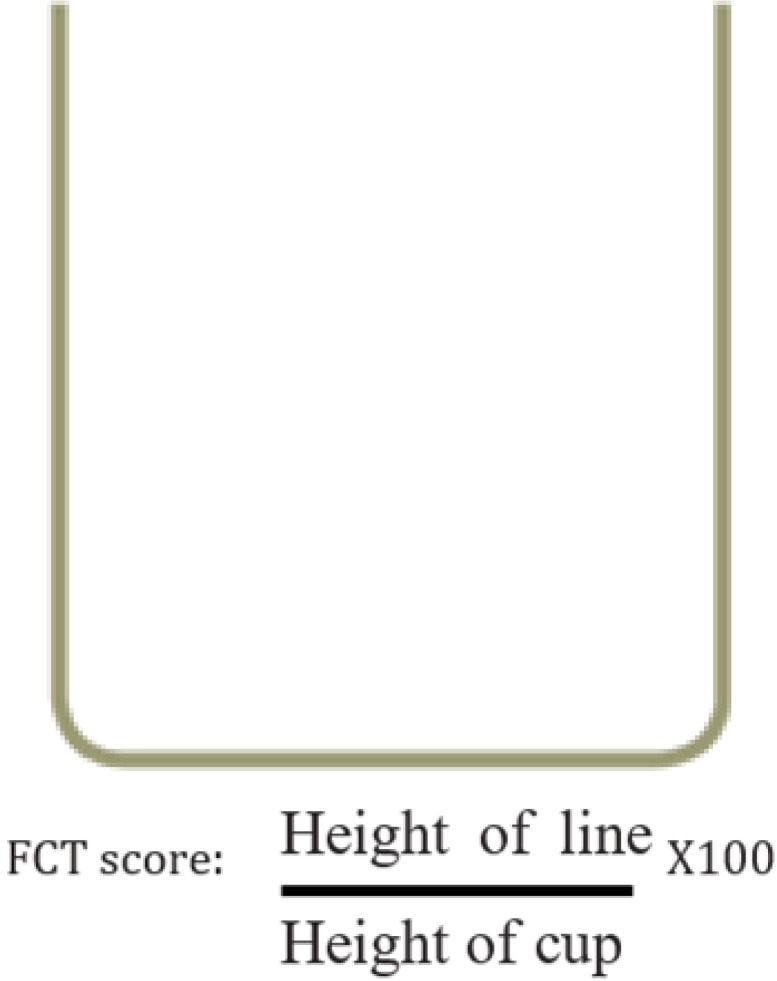
18. Ergun U, Say B, Ozer G, Yildirim O, Kocaturk O, Konar D, et al. Trial of a new pain assessment tool in patients with low education: the full cup test. Int J Clin Pract. 2007; 61:1692–1696. PMID:
17877654.
19. McCahon S, Strong J, Sharry R, Cramond T. Self-report and pain behavior among patients with chronic pain. Clin J Pain. 2005; 21:223–231. PMID:
15818074.

20. Odai ED, Ehizele AO, Enabulele JE. Assessment of pain among a group of Nigerian dental patients. BMC Res Notes. 2015; 8:251. PMID:
26087661.

21. Isik K, Unsal A, Kalayci A, Durmus E. Comparison of three pain scales after impacted third molar surgery. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011; 112:715–718. PMID:
21441046.

22. Lara-Munoz C, De Leon SP, Feinstein AR, Puente A, Wells CK. Comparison of three rating scales for measuring subjective phenomena in clinical research. I. Use of experimentally controlled auditory stimuli. Arch Med Res. 2004; 35:43–48. PMID:
15036799.
23. Gagliese L, Weizblit N, Ellis W, Chan VW. The measurement of postoperative pain: A comparison of intensity scales in younger and older surgical patients. Pain. 2005; 117:412–420. PMID:
16153776.

24. Sloman R, Wruble AW, Rosen G, Rom M. Determination of clinically meaningful levels of pain reduction in patients experiencing acute postoperative pain. Pain Manag Nurs. 2006; 7:153–158. PMID:
17145489.

25. Bech RD, Lauritsen J, Ovesen O, Overgaard S. The Verbal Rating Scale Is Reliable for Assessment of Postoperative Pain in Hip Fracture Patients. Pain Res Treat. 2015; 2015:676212. PMID:
26078880.

26. Bijur PE, Silver W, Gallagher EJ. Reliability of the visual analog scale for measurement of acute pain. Acad Emerg Med. 2001; 8:1153–1157. PMID:
11733293.

27. Bijur PE, Latimer CT, Gallagher EJ. Validation of a verbally administered numerical rating scale of acute pain for use in the emergency department. Acad Emerg Med. 2003; 10:390–392. PMID:
12670856.

28. Goodenough B, Champion GD, von Baeyer C, Ziegler JB. Facial expression scales for self-report of pain by children: rationale, validity and utility. In : Fourth International Symposium on Pediatric Pain; 1997.
29. Shih AR, von Baeyer CL. Preschool children's seriation of pain faces and happy faces in the Affective Facial Scale. Psychol Rep. 1994; 74:659–665. PMID:
8197303.

30. Miro J, Huguet A, Nieto R, Paredes S, Baos J. Evaluation of reliability, validity, and preference for a pain intensity scale for use with the elderly. J Pain. 2005; 6:727–735. PMID:
16275596.
31. Coulthard P, Patel N, Bailey E, Coulthard MB. Measuring pain after oral surgery. Oral Surg. 2014; 7:203–208.

32. Seymour RA. The use of pain scales in assessing the efficacy of analgesics in post-operative dental pain. Eur J Clin Pharmacol. 1982; 23:441–444. PMID:
7151849.

33. Ferreira-Valente MA, Pais-Ribeiro JL, Jensen MP. Validity of four pain intensity rating scales. Pain. 2011; 152:2399–2404. PMID:
21856077.

34. Bamgbose BO, Akinwande JA, Adeyemo WL, Ladeinde AL, Arotiba GT, Ogunlewe MO. Effects of co-administered dexamethasone and diclofenac potassium on pain, swelling and trismus following third molar surgery. Head Face Med. 2005; 1:11. PMID:
16274480.

35. Matijević M, Uzarević Z, Gvozdić V, Mikelić VM, Leović D, Macan D. The influence of surgical experience, type of instructions given to patients and patient sex on postoperative pain intensity following lower wisdom tooth surgery. Acta Clin Croat. 2013; 52:23–28. PMID:
23837269.
36. Fenlon S, Collyer J, Giles J, Bidd H, Lees M, Nicholson J, et al. Oral vs intravenous paracetamol for lower third molar extractions under general anaesthesia: is oral administration inferior? Br J Anaesth. 2013; 110:432–437. PMID:
23220855.

37. Raiesian S, Khani M, Khiabani K, Hemmati E, Pouretezad M. Assessment of low-level laser therapy effects after extraction of impacted lower third molar surgery. J Lasers Med Sci. 2017; 8:42–45. PMID:
28912943.

38. Nicoli GA, Conte-Neto N, Campos JADB, Cabrini-Gabrielli MA, Pereira-Filho VA. Efficacy of lumiracoxib versus diclofenac sodium in pain control following extraction of impacted lower third molar. Int J Odontostomat. 2017; 11:47–51.
39. Huskisson EC. Visual analogue scales. In : Melzack R, editor. Pain measurement and assessment. New York: Raven Press;1983. p. 33–37.
40. Silva de Oliveira JC, Grossi de Oliveira GA, Bassi AP. Comparative Assessment of the Effect of Ibuprofen and Etodolac on Edema, Limited mouth opening, and Pain in Lower Third Molar Surgery: A Randomized Clinical Trial. J Oral Maxillofac Surg. 2016; 74:1524–1530. PMID:
27160363.
41. Baxendale BR, Vater M, Lavery KM. Dexamethasone reduces pain and swelling following extraction of third molar teeth. Anaesthesia. 1993; 48:961–964. PMID:
8250191.

42. Boonsiriseth K, Latt MM, Kiattavorncharoen S, Pairuchvej V, Wongsirichat N. Dexamethasone injection into the pterygomandibular space in lower third molar surgery. Int J Oral Maxillofac Surg. 2017; 46:899–904. PMID:
28318872.
43. Aznar-Arasa L, Harutunian K, Figueiredo R, Valmaseda-Castellon E, Gay-Escoda C. Effect of preoperative ibuprofen on pain and swelling after lower third molar removal: a randomized controlled trial. Int J Oral Maxillofac Surg. 2012; 41:1005–1009. PMID:
22521671.

44. de Santana-Santos T, de Souza-Santos JA, Martins-Filho PR, da Silva LC, de Oliveira e Silva ED, Gomes AC. Prediction of postoperative facial swelling, pain and limited mouth opening following third molar surgery based on preoperative variables. Med Oral Patol Oral Cir Bucal. 2013; 18:e65–e70. PMID:
23229245.
45. Barreiro-Torres J, Diniz-Freitas M, Lago-Mendez L, Gude-Sampedro F, Gandara-Rey JM, Garcia-Garcia A. Evaluation of the surgical difficulty in lower third molar extraction. Med Oral Patol Oral Cir Bucal. 2010; 15:e869–e874. PMID:
20526272.

46. Koyuncu BÖ, Zeytinoğlu M, Çetingül E. Comparison of 2 different flap techniques in the surgical removal of bilateral impacted mandibular third molars. Turk J Med Sci. 2013; 43:891–898.

47. Nedal AM. The effects of primary and secondary wound closure following surgical extraction of lower third molars on post-operative morbidity: A prospective randomized clinical trial. J Dent Oral Hyg. 2015; 7:168–174.

48. Anighoro EO, Gbotolorun OM, Adewole RA, Arotiba GT, Effiom OA. Assessment of the effect of wound closure technique on postoperative sequaele and complications after impacted mandibular third molar extraction. Open J Stomatol. 2013; 3:527–532.

49. Gülşen U, Şentürk MF. Effect of platelet rich fibrin on edema and pain following third molar surgery: a split mouth control study. BMC Oral Health. 2017; 17:79. PMID:
28438151.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download