Abstract
Homozygous familial hypercholesterolemia (HoFH) is a rare inherited disorder that presents as abnormally elevated levels of low-density lipoprotein cholesterol and premature heart disease, requiring frequent intervention through lipid apheresis for management. The risk of perioperative cardiac events is higher in patients with HoFH because of its pathophysiological manifestations in the vascular system. Careful cardiac precautions and anesthetic assessments are necessary to ensure patient safety. In the following case report, we discuss the clinical course and anesthetic considerations for a 14-year-old girl with HoFH undergoing sedation for dental extractions and mandibular molar uprighting in an outpatient oral surgery clinic. Considerations included the use of heparin in the patient's weekly plasma lipid apheresis treatment. In order to reduce the risks of peri- and postoperative bleeding and perioperative cardiac events, the operation was scheduled for 4 days after apheresis. This allowed for adequate heparin clearance, while also reducing the likelihood of possible cardiac events. A literature review revealed no results for the outpatient management of patients with HoFH undergoing sedation for noncardiac procedures. Our reported case serves as a clinical example for physicians to be utilized in the future.
Homozygous familial hypercholesterolemia (HoFH) is an inherited metabolic disorder that clinically manifests as markedly elevated low-density lipoprotein cholesterol (LDL-C) levels [1]. Patients with HoFH are at a high risk of premature atherosclerotic plaques and cardiovascular disease even in the second or third decade of life [23]. While heterozygous familial hypercholesterolemia is common, with an approximate prevalence of 1 in 500 people, HoFH is rare, with an estimated prevalence of 1 in 1,000,000 people [145]. The affected populations are non-specific partly because of the lack of specific clinical data estimating demographic distributions of the prevalence [1].
Compared to the heterozygous variant, the observed phenotype in HoFH is more severe, with significantly higher serum LDL-C levels [16]. Mutations in the gene coding for the LDL receptor (LDLR) pathway lead to the observed phenotype; however, different portions of the LDLR pathway may be affected [67]. Other genes associated with the elevated LDL-C phenotype include the apolipoprotein B-100 (APOB), LDL receptor adaptor protein 1 (LDLRAP1), and proprotein convertase subtilin/kexin 9 (PCSK9) genes (Table 1) [16].
HoFH is diagnosed based on the results of family history, genetic testing, screening for elevated serum free LDL-C levels, and collections of cholesterol deposits, known as tendon xanthomas [137]. Patients present during childhood for examination of interdigital xanthomas, which are specifically characteristic of HoFH [7]. Laboratory findings include fasting serum LDL-C levels ≥ 500 mg/dl in untreated patients and ≥ 300 mg/dl in treated patients [17]. Examinations should be repeated to confirm the diagnosis and rule out secondary pathologies that present with elevated serum cholesterol [1]. Genetic testing helps determine defects in the implicated gene(s), thus confirming HoFH; however, it is not always necessary [78].
Anesthesiologists face many challenges in safely providing anesthetic care to patients with HoFH. The risk of perioperative cardiovascular events is markedly greater in patients with HoFH because of the underlying disease pathophysiology. Minimizing such risk is essential for the safety of patients with HoFH undergoing surgical procedures under intravenous sedation. Here, we report a case of HoFH in a teenager undergoing oral and maxillofacial surgery and describe the anesthetic and surgical management.
A 14-year-old girl was scheduled for extractions of all third molars and remaining mandibular primary teeth #K, #S, and #T and surgical uprighting of the mandibular second molars (Fig. 1). Her medical history was significant for HoFH, anxiety, depression, and gastroesophageal reflux disease. She was diagnosed with HoFH at age 13 following an exploratory cardiac catheterization. Before the diagnosis, she had never experienced a cardiac event as is common with incidental diagnoses of HoFH. Her cardiovascular family history was unknown because of adoption at 33 weeks. Following the diagnosis of HoFH, an arteriovenous fistula was placed in her right forearm to allow for vascular access during lipid apheresis, which she underwent weekly.
Extra- and intraoral findings were normal, with no temporomandibular joint clicking, popping, or pain, lymphadenopathy, facial asymmetry, or xanthomas. Risks, benefits, complications, and alternative treatments were discussed. All surgical and anesthetic options were reviewed, and the patient elected to undergo total intravenous anesthesia (TIVA) because of her anxiety and phobia of dental procedures.
Anesthetic assessment to determine the patient's candidacy for TIVA was performed because of HoFH. Preoperative medical clearance from the patient's cardiologist was obtained with no perioperative cardiac recommendations. Per the patient's cardiologist, she never exhibited any positive cardiac risk factors or symptoms that would warrant cardiac monitoring before anesthesia. The appropriate time to perform the procedure was determined such that it was in succession to her therapeutic lipid apheresis and accounted for heparin drips in concert.
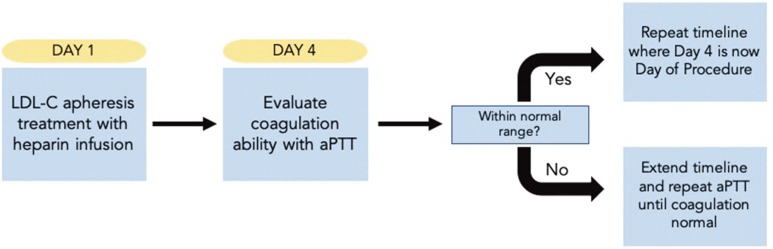
The physician overseeing the patient's apheresis recommended that the procedure be scheduled approximately for 4 days following apheresis treatment. Finally, the plan was formulated as follows: 1) The patient would receive her regular lipid apheresis treatment; 2) After 4 days, the activated partial thromboplastin time (aPTT) test would be performed to evaluate her clotting ability; 3) If the aPTT was within standard limits, the procedure would be scheduled for day 4 after her next lipid apheresis treatment (Fig. 2).
Four days after apheresis, the patient's reported aPTT value was 39.0 s, which was within a standard range of 25.9 to 39.5 s. The patient presented for surgery with a weight of 40 kg, height of 1.45 m, NPO ≥ 8 h, American Society of Anesthesiologists score III, Mallampati score III, and body mass index of 17.8 kg/m2. Monitors were placed on the patient, and intravenous access was established on the dorsum of the left hand to avoid complications associated with functioning of her arteriovenous fistula. The patient exhibited vasovagal syncope following intravenous access and was placed in the Trendelenburg position. Oxygen was delivered at 4 L/min via a nasal cannula, and a cold compress was placed on her forehead.
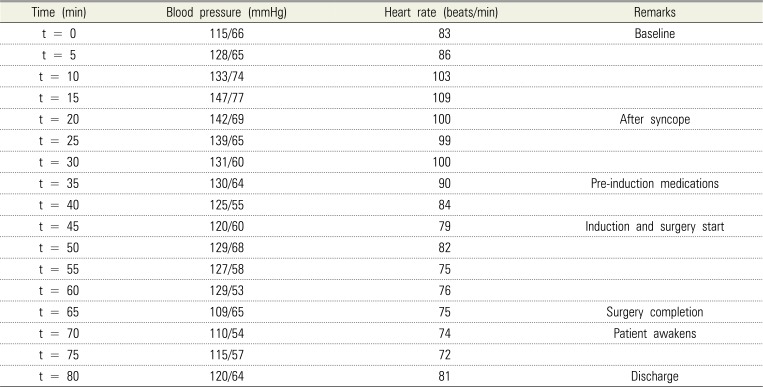
Baseline vitals before syncope were: blood pressure, 115/66 mmHg; heart rate, 83 beats/min; and oxygen saturation, 100%. After syncope, once vital signs returned to normal, intravenous doses of 1 mg midazolam, 50 µg fentanyl, 4 mg ondansetron, 4 mg dexamethasone, and 1 mg cefazolin were administered. Anesthesia was induced with an initial bolus of 40 mg of propofol, while sedation was maintained with an additional 220 mg of propofol hand-bolused in increments of 10 to 20 mg every 1 to 2 min and titrated to be effective throughout the procedure.
Local anesthesia was induced by infiltrating surgical sites with 8.5 ml of 2% lidocaine with 1 : 100,000 epinephrine. A bite block and throat pack were placed. Extractions of primary teeth and third molars were achieved with a #15 blade, followed by periosteal and straight elevators. Surgical dressings were placed at all surgical sites for hemostasis, and surgical sites were closed with a 3-0 gut suture to achieve primary closure. Surgical uprighting of both mandibular molars was achieved by introducing a small straight elevator to the mesial aspect of both teeth, without damaging the adjacent teeth or fully luxating the mandibular second molars.
The total procedure time was 22 min, and sedation lasted for 40 min. Vital signs were stable throughout the perioperative period (Table 2). Blood loss was minimal, and she was closely monitored postoperatively for excessive bleeding. The patient was arousable, and vital signs stabilized within 20% range of her initial baseline. She was awake, alert, and oriented to her surroundings, and was able to walk with minimal assistance. The patient returned to the clinic 1 week after the procedure and reported good wound healing at operative sites with minimal pain and swelling.
Reduction in serum LDL-C in patients with HoFH necessitates more aggressive treatment modalities than traditional statin therapies and lifestyle changes [234]. Lipid apheresis is effective; however, it must be performed weekly or biweekly to maintain normal serum LDL-C levels, or serum values return to pretreatment levels between 2–4 weeks following apheresis [34].
Different techniques are used in removal of lipoproteins: immunoadsorption, dextran sulphate-cellulose adsorption, heparin extracorporeal LDL precipitation systems, and direct adsorption of lipoprotein using hemoperfusion [9]. Our patient utilized the heparin extracorporeal LDL precipitation system technique, which was important consider when selecting the date of procedure given the bleeding risks associated with heparin usage.
Date selection for our patient was important for two primary reasons. First, we wanted to minimize the patient's risk for a cardiovascular event by choosing a date that immediately followed apheresis treatment to ensure optimal cardiovascular function. Second, although the ideal date of surgery would have been the day following apheresis, she received heparin infusions at each weekly treatment, inducing risk for excessive bleeding during the peri- and postoperative periods. This could hinder the procedure and subsequent healing at the incision sites. The physician supervising our patient's apheresis treatment recommended a waiting period of 4 days in addition to evaluating aPTT in order to confirm she has acceptable clotting ability.
Cardiac evaluation is necessary before surgery to thoroughly assess risk in patients with HoFH. Measures include evaluating the cardiac functional status through questionnaires, stress testing, performing resting echocardiography, assessing troponin and brain natriuretic peptide values, and even performing cardiac catheterization [10]. The type of noncardiac surgery and degree of invasiveness also determine how the patient should be evaluated from a cardiovascular standpoint before procedure [10].
To the best of our knowledge, this is the first report describing the management of a patient with HoFH undergoing an elective, noncardiac surgery under intravenous sedation. Rarity of the diseases is as a potential roadblock in the anesthetic management of patients with HoFH; however, in our report, we have provided a framework that clinicians may utilize for patients with HoFH requiring treatment in the oral surgery practice.
Notes
AUTHOR CONTRIBUTIONS:
Saad Khan: Conceptualization, Writing – original draft, Writing – review & editing.
Samuel Min: Visualization, Writing – original draft.
Garrett Willard: Data curation, Writing – original draft.
Iris Lo: Conceptualization, Supervision.
Rachael D'Souza: Conceptualization, Supervision.
Aaron Park: Conceptualization, Supervision, Writing – review & editing.
References
1. Singh S, Bittner V. Familial hypercholesterolemia--epidemiology, diagnosis, and screening. Curr Atheroscler Rep. 2015; 17:482. PMID: 25612857.

2. Gidding SS. Managing patients with homozygous familial hypercholesterolemia. J Am Coll Cardiol. 2017; 70:1171–1172. PMID: 28838367.

3. Robinson JG. Management of familial hypercholesterolemia: a review of the recommendations from the National Lipid Association Expert Panel on Familial Hypercholesterolemia. J Manag Care Pharm. 2013; 19:139–149. PMID: 23461430.

4. Cuchel M, Bruckert E, Ginsberg HN, Raal FJ, Santos RD, Hegele RA, et al. Homozygous familial hypercholesterolaemia: new insights and guidance for clinicians to improve detection and clinical management. A position paper from the Consensus Panel on Familial Hypercholesterolaemia of the European Atherosclerosis Society. Eur Heart J. 2014; 35:2146–2157. PMID: 25053660.

5. Ladha S, Makhija N, Kiran U, Aarav SK. Homozygous familial hypercholesterolemia: anesthetic challenges and review of literature. World J Pediatr Congenit Heart Surg. 2017; 2150135117702127. PMID: 28952401.

6. Raal FJ, Hovingh GK, Catapano AL. Familial hypercholesterolemia treatments: guidelines and new therapies. Atherosclerosis. 2018; 277:483–492. PMID: 30270089.

7. Raal FJ, Santos RD. Homozygous familial hypercholesterolemia : current perspectives on diagnosis and treatment. Atherosclerosis. 2012; 223:262–268. PMID: 22398274.
8. Cartier JL, Goldberg AC. Familial hypercholesterolemia: advances in recognition and therapy. Prog Cardiovasc Dis. 2016; 59:125–134. PMID: 27477957.

10. Cohn SL, Fleisher LA. Evaluation of cardiac risk prior to noncardiac surgery. Waltham, MA: UpToDate;2019.
11. Shapiro MD, Fazio S. Apolipoprotein B-containing lipoproteins and atherosclerotic cardiovascular disease. F1000Res. 2017; 6:134. PMID: 28299190.

Fig. 1
The panoramic radiograph shows impacted but not clinically erupted third molars, clinically erupted primary teeth close to exfoliation, and palpable mandibular second molars that are not entirely erupted.
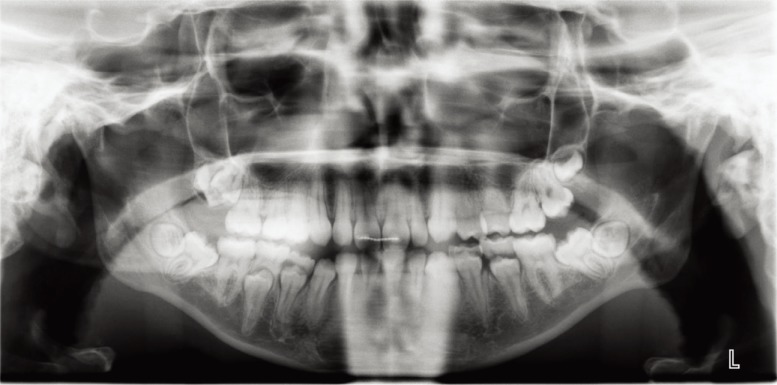
Table 1
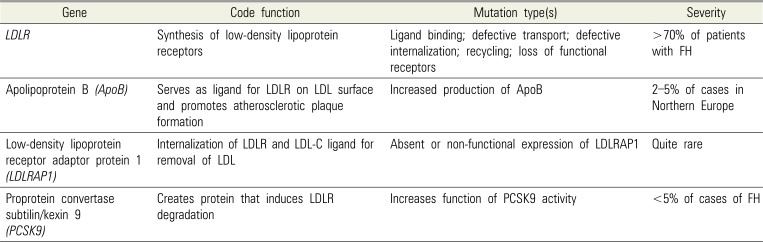
Table 2
Patient's vital signs during the pre-, peri-, and postoperative periods




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download