Abstract
Purpose
To report an initial single-surgeon experience with single-port robot-assisted radical prostatectomy (SP-RARP) using the da Vinci SP surgical system (Intuitive Surgical, USA).
Materials and Methods
Between December 2018 and October 2019, a single surgeon performed SP-RARP in 20 patients with prostate cancer. SP-RARP was performed using the conventional approach through an umbilical port with a GelPOINT access system (Applied Medical, USA) and an additional assist port. During surgery, the camera was placed in the 6- or 12-o'clock position, and a traction arm was placed in the counterpart position for upward or downward traction. Clinicopathologic data, perioperative data, and short-term surgical outcomes were analyzed.
Results
Of 20 patients, 45% of patients had pT3 or greater disease and 45% had Gleason grade 4 to 5, respectively. In 11 patients that underwent lymph node dissection, the median number of lymph nodes removed was 19 (interquartile range [IQR], 14–22). Median operative time was 245 minutes (IQR, 200–255), and median console time was 190 minutes (IQR, 165–210). Median blood loss was 200 mL (IQR, 150–300 mL), and there were no intraoperative complications or open conversion. In 10 patients with a follow-up period longer than 3 months, one patient experienced biochemical recurrence, and all patients required 0 to 1 pads per day. Of seven patients that were potent before surgery, four recovered erectile function sufficient for intercourse.
Radical prostatectomy is the primary treatment approach for localized prostate cancer. After the introduction of the robotic surgical system, robotics has been rapidly adopted for radical prostatectomy. More than 80% of radical prostatectomies in the United States are performed robotically [1]. Additionally, robot-assisted radical prostatectomy (RARP) represents more than 50% of all radical prostatectomies performed in Korea [2]. Recent meta-analyses have shown significant advantages of RARP compared with the conventional open technique in terms of urinary continence and recovery of potency [34].
Robotic laparoendoscopic single-site surgery (LESS) was first described by Kaouk et al. [5] in 2009. However, there are still intrinsic limitations even with the da Vinci Si and Xi platforms (Intuitive Surgical, Sunnyvale, CA, USA). Particularly, radical prostatectomy with a single-port approach is a challenging surgical procedure. Although a limited number of LESS radical prostatectomies have been reported, this technique has not been widely adopted by urologists because of technical difficulties.
The da Vinci SP system (Intuitive Surgical) is specifically designed for single-port surgery. This system includes a 12×10-mm articulating robotic camera and three 6-mm, double-jointed, articulating robotic instruments. Initial experiences with single-port RARP (SP-RARP) using the da Vinci SP system have recently been reported [678]. In Korea, the da Vinci SP system received regulatory clearance in August 2018. We performed the first SP-RARP using the da Vinci SP in December 2018. The purpose of this study was to report our first clinical experience with the da Vinci SP system for performing radical prostatectomy.
Between December 2018 and October 2019, a single surgeon (KHK) performed SP-RARP in 20 consecutive patients with prostate cancer. The surgeon had experience with >100 cases of conventional RARP. The surgeon received intensive training on the SP system using an animal model for robotic surgical simulation. This was a retrospective study, approved by the Institutional Review Board of Ewha Womans University Seoul Hospital (approval number: 2019-05-014). All study protocols were carried out in accordance with the Declaration of Helsinki. Various parameters were analyzed, including clinical and pathologic data, perioperative outcomes of operative time and estimated blood loss, intraoperative or perioperative complications, and surgical outcomes.
A 2.5- to 3-cm skin incision was made in the umbilicus, and a GelPOINT access system (Applied Medical, Ranch Santa Margarita, CA, USA) was used for SP port insertion. An additional 10-mm assistant port was inserted in the left lower quadrant (Fig. 1). The surgery started with the camera placed at the 6-o'clock position. The posterior peritoneum was incised, and seminal vesicles and the vas deferens were dissected. Posterior dissection was performed with upward traction of the seminal vesicles and the vas deferens (Fig. 2A). The camera position was changed to 12 o'clock, and the median umbilical ligament was transected to develop a Retzius space with two robotic arms (Fig. 2B). If the distance was not sufficient for robotic arm triangulation to transect the umbilical ligament, the SP port was pulled and the remote center of the SP port was placed outside the skin. This approached helped to maximize the distance between the SP port and the working space. Lymph node dissection was usually performed with the standard template including external, obturator, and internal iliac packets. The endopelvic fascia was incised. The bladder was retracted cephalad using Cadiere forceps in the 6-o'clock position, and the vesicoprostatic junction was dissected. The urethral catheter was retracted anteriorly by the assistant or by using an endoclosure device and 1-0 Vicryl sutures (Fig. 2C). After the posterior bladder neck was dissected, the camera was placed at 6 o'clock, and the retrotrigonal fascia was dissected to expose the seminal vesicles and vas deferens with upward traction of the prostate using a robotic arm in the 12-o'clock position (Fig. 2D). A posterior and lateral dissection was performed for neurovascular bundle preservation. The camera view of the surgical field could be restricted when the seminal vesicle was retracted in the direction opposite to neurovascular bundle dissection. In this situation, the procedures can be performed by adjusting the field of vision with manipulation of the flexible camera (Fig. 2E). When the posterior dissection approached the apex, the camera was placed at 12 o'clock, and the lateral aspect of the apex was dissected. With cephalad retraction of the prostate, the dorsal vein complex was transected with cautery, and the urethra was exposed. Urethral dissection was performed by rotating the prostate to assess the shape of the apex, and the urethra was transected by cold scissors (Fig. 2F). Vesicourethral anastomosis used only the two robotic arms of the needle driver and barbed suture. Posterior reconstruction was performed, and a running suture was used for watertight anastomosis (Fig. 2G). For anterior reconstruction and to control bleeding of the dorsal vein complex, the anterior bladder neck muscle fiber and parietal layer of the pelvic fascia were approximated (Fig. 2G). A drain was placed through an assist port, and the specimen bag was removed through the SP port site without additional incision (Video clip, Supplementary material).
Clinicopathologic data are summarized in Table 1. Of 20 patients, 9 (45.0%) and 8 (40.0%) had intermediate and highrisk prostate cancer, respectively. In the final pathology results, 45% of patients had pT3 or higher lesions and 45% had Gleason grade 4 to 5, respectively. Seven patients (35.0%) had positive surgical margins. A total of 18.2% (2/11) and 55.6% (5/9) of patients with pT2 and ≥pT3 cancer had positive surgical margins, respectively. In 11 patients treated with lymph node dissection, the median number of lymph nodes removed was 19 (interquartile range [IQR], 14–22), and 2 patients had a positive lymph node.
Perioperative outcomes are summarized in Table 2. Median operative time was 245 minutes (IQR, 200–255 minutes), and median console time was 190 minutes (IQR, 165–210 minutes). In patients who were not treated with lymph node dissection, median console time was 165 minutes (IQR, 140–180 minutes). Fig. 3 shows the decreasing console time in patients without lymph node dissection. Median estimated blood loss was 200 mL (IQR, 150–300 mL), and none of the patients received a transfusion. There were no intraoperative or postoperative complications of Clavien grade >2. No cases required additional ports, multiport RARP, or open conversion. The urethral catheter was removed within 8 days, with the exception of one patient who had a urinary leak on the cystogram and experienced catheter removal on day 14.
In 10 patients with a follow-up period greater than 3 months, none of the patients experienced biochemical recurrence except for 1 patient who had pT3N1 and Gleason grade 5 disease. Eight patients did not use a pad, and two used one pad per day for safety. Of seven patients who were potent before surgery, four recovered erectile function sufficient for intercourse with or without a phosphodiesterase-5 inhibitor, and they were all aged 60 years or younger.
Since LESS was first described by Kaouk et al. [5], it has been applied in various urological surgeries. Despite initial enthusiasm for LESS, it has received consistent criticism for lack of triangulation and instrument clashing. Numerous efforts have been made to overcome these technical limitations, and the robotic system has contributed to fundamental improvements in this field. Nevertheless, robotic LESS is a developing technique with limited use, mainly as the result of technical difficulties and a steep learning curve. In addition, most robotic LESS has been performed in renal lodge procedures, and the technique has not become popular in radical prostatectomy [910]. The limited use of robotic LESS in radical prostatectomy is not only due to technical difficulties and the steep learning curve, but also to inherent limitations such as the lack of the EndoWrist technology (Intuitive Surgical), insufficient mechanical force, and the absence of a third arm. These limitations are closely related to surgical outcomes in radical prostatectomy because they affect important surgical procedures such as neurovascular bundle preservation, apical dissection, and urethrovesical anastomosis.
However, development of a purpose-built single-port robotic platform is likely to overcome most of these limitations. The da Vinci SP platform has 7 degrees of wrist movement and an additional elbow joint for triangulation. In our experience, the da Vinci SP platform provides capabilities and instrument maneuverability similar to those of the multiport da Vinci platform. Although the Si or Xi single-port platforms have two robotic curved and semirigid instruments, the da Vinci SP platform has three robotic arms, and high force transmission is possible with a novel elbow joint mechanism. These advantages facilitate precise tissue handling, meticulous dissection, and tissue traction using a third arm and suturing, which are critical during radical prostatectomy. Although no objective data are available comparing mechanical force with the da Vinci SP and multiport instruments, we experienced that the mechanical force was a little weak when the apical portion of the neurovascular bundle was dissected. Thus, we used both sharp and blunt dissection for this part. Although this is an initial experience with short-term follow-up, the functional outcomes were satisfactory: all patients aged 60 years or younger recovered their erectile function, and all patients with follow-up of more than 3 months used 0 or 1 pad for safety. In this series, one patient had locally advanced disease and had multiple enlarged lymph nodes and bladder neck invasion. The final pathology was pT4N1 (lymph node yield, 22; number of positive lymph nodes, 9). Even though there were no intraoperative complications, it took more than 2 hours for lymph node dissection, and total console time was 340 minutes. We do not believe that the da Vinci SP system should not be applied to high-risk prostate cancer. However, we suggest that surgeons start this surgery in clinically localized prostate cancer.
We found some characteristics of the da Vinci SP system to differ from the conventional multiport system. First, the da Vinci SP system has limitations in the distance from the port incision site to the target anatomy. For instrument triangulation and separation of an additional elbow joint, the target anatomy should be at least 10 cm from the trocar. Thus, peritoneal incision and transection of the umbilical ligament would be difficult with the umbilical port. Whereas previous SP-RARP used a supra-umbilical incision for SP port insertion [671112], we created an umbilical port incision for scarless surgery. We used the GelPOINT system for port placement, and the remote center of the SP port could be placed outside of the skin incision with the GelPOINT system. This procedure creates additional distance and working space during SP-RARP. Second, the working space is limited, because the distance between each robotic arm is about 10 cm. However, the da Vinci SP system is well suited for radical prostatectomy because the prostate is a small organ and most surgical procedures are performed in a pelvic space. Also, we can maximize the surgical working space and the operative visual field if we properly use articulation of the endoscopic camera.
We recently summarized a published series of SP-RARP as shown in Table 3, and our series showed comparable operative time and EBL [6111314]. There were no major operative complications or open conversion during SP-RARP in either the previous reports or our series, indicating that SP-RARP is a safe procedure. Although the positive margin rate was relatively high, the patients included in this study had unfavorable pathologic features. The proportions of patients with ≥pT3 and Gleason grade 4 to 5 were 45% and 45%, respectively. The initial learning curve for this technique and unfavorable pathologic features are likely to contribute to a relatively higher rate of positive surgical margins.
For SP-RARP, surgeries were planned according to the overall direction of traction: upward or downward. Depending on the direction of traction, the camera was positioned at 6 or 12 o'clock, with the Cadiere forceps in the counter position. For example, posterior and lateral dissection of the prostate was performed with upward traction by use of Cadiere forceps at 12 o'clock and a camera at 6 o'clock. Next, the camera was positioned in the 12-o'clock position, and apical dissection and urethral transection were performed with downward traction of the prostate with Cadiere forceps in the 6-o'clock position. From our experience, surgical planning that depends on upward or downward traction is very important for efficient robotic arm movement.
There were some limitations to this study. The number of patients included was small for statistical analysis. Our institute established a new hospital with installation of the da Vinci SP system, and the surgical team and surgical assistant did not have extensive experience with the robotic system, which could have affected surgical time and surgical outcomes. However, a well-trained representative from Intuitive Surgical attended the initial cases, and the surgical team and assistant stably adapted to the new surgical system without intraoperative complications. Although this study showed the safety and feasibility of SP-RARP, a larger number of patients and a comparative cohort treated by multiport RARP are necessary in subsequent study.
In this study, we report an initial, single-surgeon experience with SP-RARP. We described the surgical procedure for SP-RARP based on our experience. This is the first report of clinical experience with SP-RARP in Korea. Our report showed the safety and feasibility of SP-RARP and that surgical outcomes on the basis of short-term follow-up were satisfactory.
References
1. Oberlin DT, Flum AS, Lai JD, Meeks JJ. The effect of minimally invasive prostatectomy on practice patterns of American urologists. Urol Oncol. 2016; 34:255.e1–255.e5.

2. Park J, Suh B, Shin DW, Hong JH, Ahn H. Changing patterns of primary treatment in Korean men with prostate cancer over 10 years: a nationwide population based study. Cancer Res Treat. 2016; 48:899–906. PMID: 26511804.

3. Ficarra V, Novara G, Rosen RC, Artibani W, Carroll PR, Costello A, et al. Systematic review and meta-analysis of studies reporting urinary continence recovery after robot-assisted radical prostatectomy. Eur Urol. 2012; 62:405–417. PMID: 22749852.

4. Ficarra V, Novara G, Ahlering TE, Costello A, Eastham JA, Graefen M, et al. Systematic review and meta-analysis of studies reporting potency rates after robot-assisted radical prostatectomy. Eur Urol. 2012; 62:418–430. PMID: 22749850.

5. Kaouk JH, Goel RK, Haber GP, Crouzet S, Stein RJ. Robotic single-port transumbilical surgery in humans: initial report. BJU Int. 2009; 103:366–369. PMID: 18778353.

6. Agarwal DK, Sharma V, Toussi A, Viers BR, Tollefson MK, Gettman MT, et al. Initial experience with da vinci single-port robot-assisted radical prostatectomies. Eur Urol. 2019; 4. 19. DOI: 10.1016/j.eururo.2019.04.001. [Epub].

7. Kaouk J, Bertolo R, Eltemamy M, Garisto J. Single-port robot-assisted radical prostatectomy: first clinical experience using the sp surgical system. Urology. 2019; 124:309. PMID: 30367924.

8. Kaouk J, Garisto J, Bertolo R. Robotic urologic surgical interventions performed with the single port dedicated platform: first clinical investigation. Eur Urol. 2019; 75:684–691. PMID: 30522914.

9. Bertolo R, Garisto J, Gettman M, Kaouk J. Novel system for robotic single-port surgery: feasibility and state of the art in urology. Eur Urol Focus. 2018; 4:669–673. PMID: 29914841.

10. Nelson RJ, Chavali JSS, Yerram N, Babbar P, Kaouk JH. Current status of robotic single-port surgery. Urol Ann. 2017; 9:217–222. PMID: 28794585.

11. Dobbs RW, Halgrimson WR, Madueke I, Vigneswaran HT, Wilson JO, Crivellaro S. Single-port robot-assisted laparoscopic radical prostatectomy: initial experience and technique with the da Vinci(®) SP platform. BJU Int. 2019; 124:1022–1027. PMID: 31301693.
12. Ng CF, Chan ESY, Teoh JYC. The use of the da Vinci SP system for retzius-sparing radical prostatectomy in cadaveric model. Urology. 2019; 125:260. PMID: 30580003.

13. Kaouk J, Valero R, Sawczyn G, Garisto J. Extraperitoneal single-port robot-assisted radical prostatectomy: initial experience and description of technique. BJU Int. 2019; 8. 06. DOI: 10.1111/bju.14885. [Epub].

14. Ng CF, Teoh JY, Chiu PK, Yee CH, Chan CK, Hou SS, et al. Robot-assisted single-port radical prostatectomy: a phase 1 clinical study. Int J Urol. 2019; 26:878–883. PMID: 31257704.
SUPPLEMENTARY MATERIAL
The supplementary video clip will be available on You-Tube: https://youtu.be/_72_rk5oQak.

Fig. 1
(A) Umbilical incision for port placement, (B) final incision after surgery, (C) and (D) port placement for single-port robot-assisted radical prostatectomy (SP-RARP). The assist port should be place at least 7 cm from the SP port, and the working space should be within 10 to 25 cm from the SP port for robotic arm triangulation.
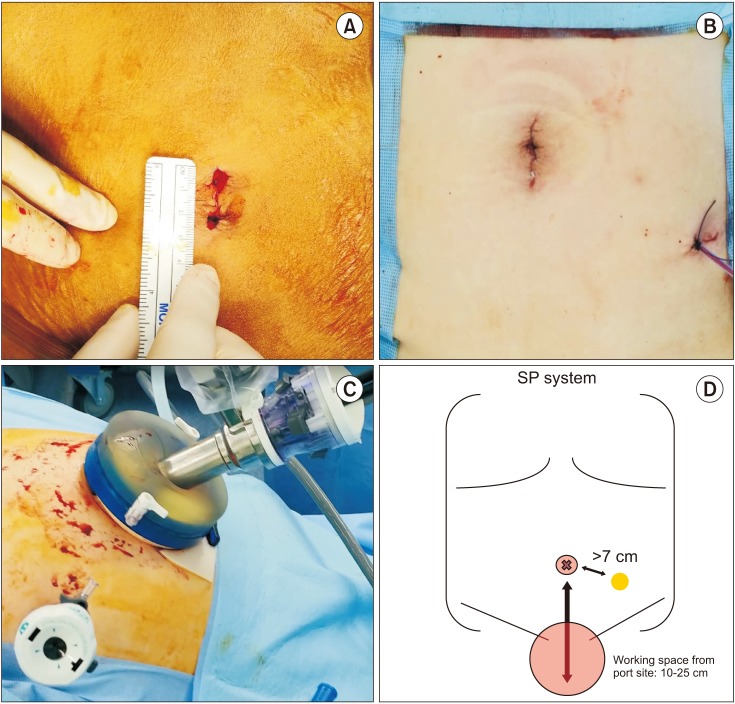
Fig. 2
The single-port robot-assisted radical prostatectomy (SP-RARP) surgical procedures. The camera was placed in the 6-o'clock position during (A) seminal vesicle dissection, (D) posterior bladder neck dissection, and (E) neurovascular bundle dissection with upward traction created by Cadiere forceps in the 12-o'clock position. (B) Umbilical ligament transection, (C) bladder neck transection, (F) apex dissection, and (G) urethrovesical anastomosis were performed with the camera positioned at 12 o'clock.
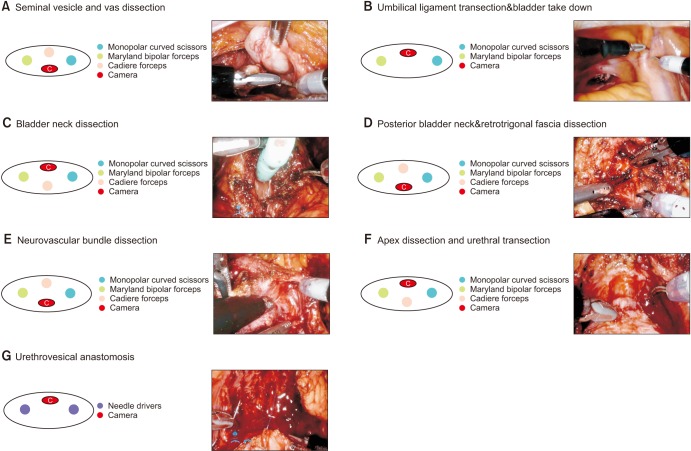
Fig. 3
(A) Console time and (B) estimated blood loss for single-port robot-assisted radical prostatectomy. PLND, pelvic lymph node dissection; EBL, estimated blood loss.

Table 1
Clinicopathologic data of 20 patients
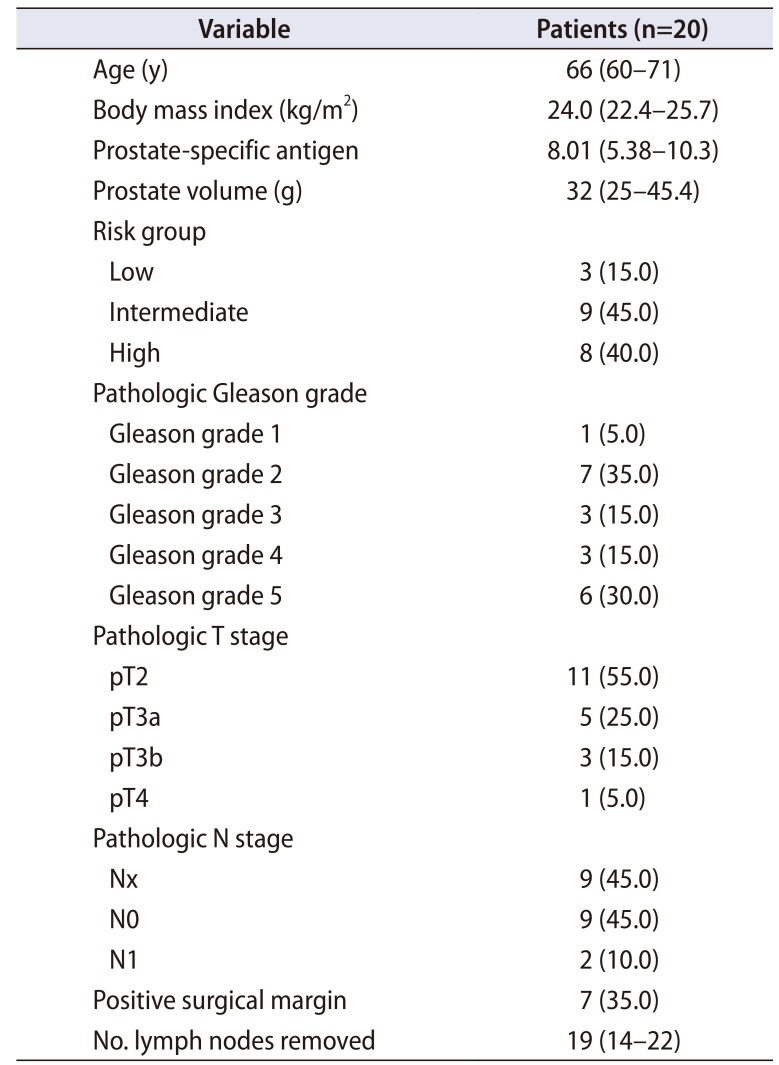
Table 2
Perioperative outcomes

Table 3
Comparison with other SP-RARP series

| Study | No. of patients | Approach | Assist port | No. of surgeons | PSA (ng/mL) | pT stage ≥3 | pGG ≥4 | LND | No. of LNs removed | PSM | Operative time (min) | EBL (mL) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Agarwal et al. [6] | 49 | Transperitoneal Retzius sparing | Plus one | 3 | 6.4 (median) | 7 (14.3) | 6 (12.2) | 36 (73.5) | 8 (median) | 13 (26.5) | 161 (median) | 200 (median) |
| Ng et al. [14] | 20 | Transperitoneal | Plus one | 2 | 15.3 (mean) | 9 (45.0) | 4 (20.0) | 6 (30.0) | 8.3 (mean) | 11 (55.0) | 208 (mean) | 296 (mean) |
| Dobbs et al. [11] | 10 | Transperitoneal | Plus one | 1 | 11.0 (mean) | 4 (40.0) | N/A | 4 (40.0) | N/A | 5 (50.0) | 234 (median) | 65 (mean) |
| Kaouk et al. [13] | 10 | Extraperitoneal | Pure sing port | 1 | 9 (mean) | 6 (60.0) | N/A | 10 (100.0) | 10.1 (mean) | 5 (50.0) | 197 (median) | 143 (mean) |
| Present study | 20 | Transperitoneal | Plus one | 1 | 8.01 (median) | 9 (45.0) | 9 (45.0) | 11 (55.0) | 19 (median) | 7 (35.0) | 245 (median) | 200 (median) |




 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download