1. Butturini G, Stocken DD, Wente MN, Jeekel H, Klinkenbijl JH, Bakkevold KE, et al. Pancreatic Cancer Meta-Analysis Group. Influence of resection margins and treatment on survival in patients with pancreatic cancer: meta-analysis of randomized controlled trials. Arch Surg. 2008; 143:75–83. discussion 83. PMID:
18209156.
2. Konstantinidis IT, Warshaw AL, Allen JN, Blaszkowsky LS, Castillo CF, Deshpande V, et al. Pancreatic ductal adenocarcinoma: is there a survival difference for R1 resections versus locally advanced unresectable tumors? What is a “true” R0 resection? Ann Surg. 2013; 257:731–736. PMID:
22968073.
3. Hartwig W, Vollmer CM, Fingerhut A, Yeo CJ, Neoptolemos JP, Adham M, et al. International Study Group on Pancreatic Surgery. Extended pancreatectomy in pancreatic ductal adenocarcinoma: definition and consensus of the International Study Group for Pancreatic Surgery (ISGPS). Surgery. 2014; 156:1–14. PMID:
24856668.

4. Loveday BPT, Zilbert N, Serrano PE, Tomiyama K, Tremblay A, Fox AM, et al. Neoadjuvant therapy and major arterial resection for potentially reconstructable arterial involvement by stage 3 adenocarcinoma of the pancreas. HPB (Oxford). 2019; 21:643–652. PMID:
30471960.

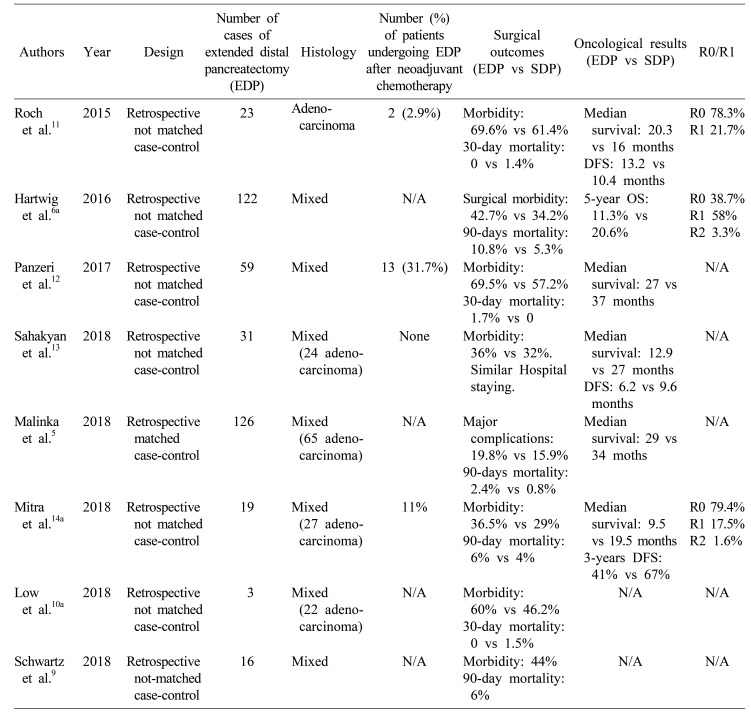
5. Malinka T, Klein F, Andreou A, Pratschke J, Bahra M. Distal pancreatectomy combined with multivisceral resection is associated with postoperative complication rates and survival comparable to those after standard procedures. J Gastrointest Surg. 2018; 22:1549–1556. PMID:
29748738.

6. Hartwig W, Gluth A, Hinz U, Koliogiannis D, Strobel O, Hackert T, et al. Outcomes after extended pancreatectomy in patients with borderline resectable and locally advanced pancreatic cancer. Br J Surg. 2016; 103:1683–1694. PMID:
27686238.

7. Bassi C, Marchegiani G, Dervenis C, Sarr M, Abu Hilal M, Adham M, et al. International Study Group on Pancreatic Surgery (ISGPS). The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 years after. Surgery. 2017; 161:584–591. PMID:
28040257.
8. Bhayani NH, Enomoto LM, James BC, Ortenzi G, Kaifi JT, Kimchi ET, et al. Multivisceral and extended resections during pancreatoduodenectomy increase morbidity and mortality. Surgery. 2014; 155:567–574. PMID:
24524390.

9. Schwartz PB, Roch AM, Han JS, Vaicius AV, Lancaster WP, Kilbane EM, et al. Indication for en bloc pancreatectomy with colectomy: when is it safe? Surg Endosc. 2018; 32:428–435. PMID:
28664444.

10. Low TY, Koh YX, Teo JY, Goh BKP. Short-term outcomes of extended pancreatectomy: a single-surgeon experience. Gastrointest Tumors. 2018; 4:72–83. PMID:
29594108.

11. Roch AM, Singh H, Turner AP, Ceppa EP, House MG, Zyromski NJ, et al. Extended distal pancreatectomy for pancreatic adenocarcinoma with splenic vein thrombosis and/or adjacent organ invasion. Am J Surg. 2015; 209:564–569. PMID:
25547091.

12. Panzeri F, Marchegiani G, Malleo G, Malpaga A, Maggino L, Marchese T, et al. Distal pancreatectomy associated with multivisceral resection: results from a single centre experience. Langenbecks Arch Surg. 2017; 402:457–464. PMID:
27787606.

13. Sahakyan MA, Kleive D, Kazaryan AM, Aghayan DL, Ignjatovic D, Labori KJ, et al. Extended laparoscopic distal pancreatectomy for adenocarcinoma in the body and tail of the pancreas: a single-center experience. Langenbecks Arch Surg. 2018; 403:941–948. PMID:
30417281.

14. Mitra A, Pai E, Dusane R, Ranganathan P, DeSouza A, Goel M, et al. Extended pancreatectomy as defined by the ISGPS: useful in selected cases of pancreatic cancer but invaluable in other complex pancreatic tumors. Langenbecks Arch Surg. 2018; 403:203–212. PMID:
29362882.

15. Shubert CR, Bergquist JR, Groeschl RT, Habermann EB, Wilson PM, Truty MJ, et al. Overall survival is increased among stage III pancreatic adenocarcinoma patients receiving neoadjuvant chemotherapy compared to surgery first and adjuvant chemotherapy: an intention to treat analysis of the National Cancer Database. Surgery. 2016; 160:1080–1096. PMID:
27522556.

16. Michelakos T, Pergolini I, Castillo CF, Honselmann KC, Cai L, Deshpande V, et al. Predictors of resectability and survival in patients with borderline and locally advanced pancreatic cancer who underwent neoadjuvant treatment with FOLFIRINOX. Ann Surg. 2019; 269:733–740. PMID:
29227344.

17. Choi JG, Nipp RD, Tramontano A, Ali A, Zhan T, Pandharipande P, et al. Neoadjuvant FOLFIRINOX for patients with borderline resectable or locally advanced pancreatic cancer: results of a decision analysis. Oncologist. 2019; 24:945–954. PMID:
30559125.

18. Moningi S, Dholakia AS, Raman SP, Blackford A, Cameron JL, Le DT, et al. The role of stereotactic body radiation therapy for pancreatic cancer: a single-institution experience. Ann Surg Oncol. 2015; 22:2352–2358. PMID:
25564157.

19. Kaiser J, Hackert T, Büchler MW. Extended pancreatectomy: does it have a role in the contemporary management of pancreatic adenocarcinoma? Dig Surg. 2017; 34:441–446. PMID:
28700995.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download