CASE
The patient was a 64-year old man, with remote history of trans-hiatal esophagectomy for the treatment of esophageal adenocarcinoma. 12 years later, he developed a pancreatic adenocarcinoma and was treated with subtotal pancreatectomy. His post-resection course was complicated with pancreatic leak, which precipitated intra-abdominal infection and left pleural empyema, which were drained. One-month post-resection, the patient presented with sanguineous drain output. He subsequently underwent arteriography, which revealed a pseudoaneurysm arising from the first segmental artery off the superior mesenteric artery (SMA) and the inferior pancreaticoduodenal artery anastomosing with the gastroduodenal artery and the gastroepiploic artery. The branch giving rise to the pseudoaneurysm was the only vessel supplying the gastric conduit. The pseudoaneurysm was a sidewall type associated with slow extravasation (
Fig. 1), and preserved flow to the gastric conduit. Due to its small diameter, the vessel would not admit stent; vessel embolization would risk gastric conduit necrosis.
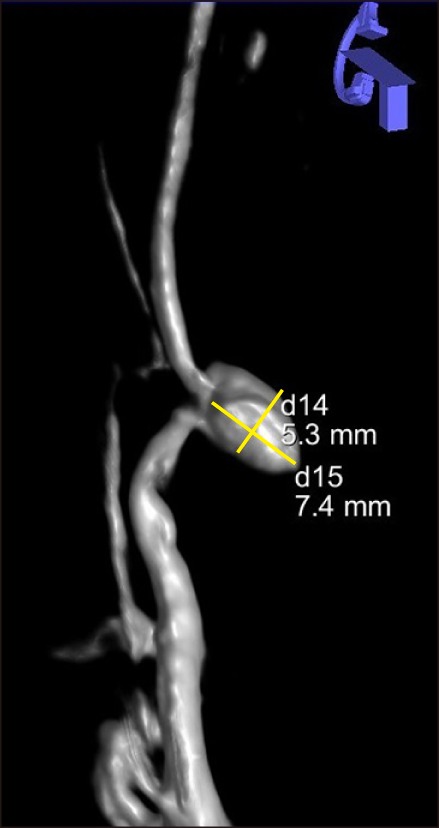 | Fig. 13-dimensional reconstruction of superior mesenteric artery (SMA) arteriography. The angiogram revealed a sidewall type pseudoaneurysm arising from the 1st segmental artery off of the SMA and the inferior pancreaticoduodenal artery (IPDA) anastomosing with the gastroduodenal artery and the gastroepiploic artery. The pseudoaneurysm dimensions were 5.3×7.4 mm.
|
During esophagectomy, the intrathoracic, distal esophagus and proximal stomach had been removed, and the residual stomach mobilized to reach the residual esophageal stump in the neck. The gastric conduit was completely dependent on blood flow from the right gastroepiploic artery (
Fig. 2). It was, therefore, decided to pursue flow-diverting stent device placement across the pseudoaneurysm to preserve gastric conduit flow. The SMA was catheterized (
Fig. 3). An intermediate distal access catheter (Navien 5F, Medtronic, Irvine CA) and a Phenom 027 microcatheter (Medtronic Irvine CA) were advanced over a Synchro 014 microwire (Stryker Neurovascular, Freemont CA) through the guide catheter. Attempts to advance this combination to the first order SMA branch supplying the pseudoaneurysm failed due to marked tortuosity. Angioplasty was performed and an Enterprise 4 mm×20 mm stent (Codman Neurovascular, Miami Lakes FL) was placed into the affected branch.
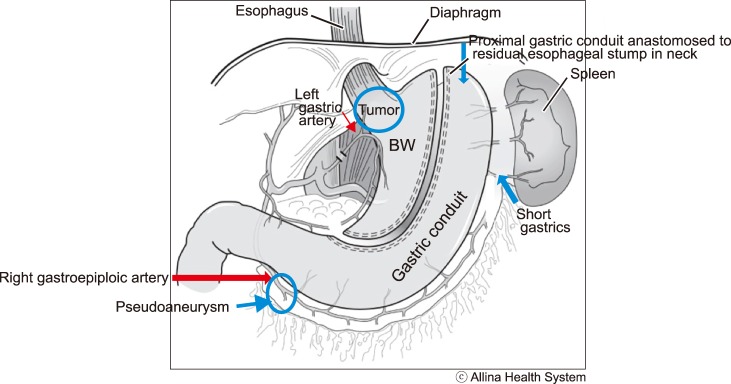 | Fig. 2Graph representation of the arterial supply of the gastric conduit. The gastric conduit was completely dependent on blood flow from the right gastroepiploic artery. The branch giving rise to the pseudoaneurysm was observed to be the only vessel supplying the gastric conduit.
|
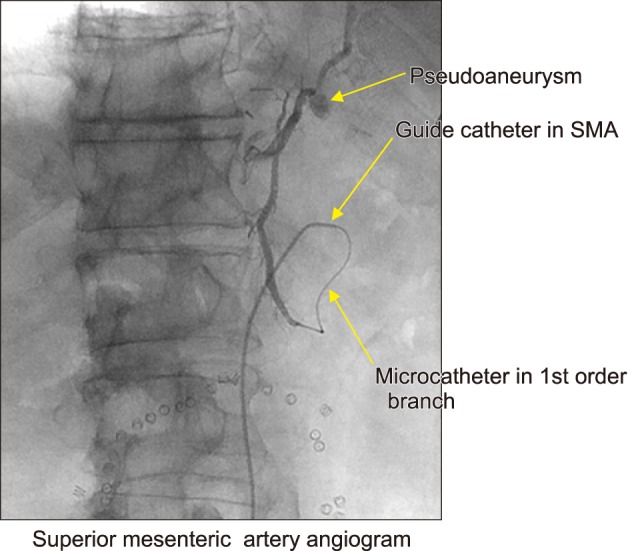 | Fig. 3Superior mesenteric artery angiogram. After catheterization of the SMA, a microcatheter was advanced to the 1st order SMA branch. Injection of contrast revealed the sidewall pseudoaneurysm. This SMA branch appeared to be supplying the only vessel shown to be supplying the gastric conduit.
|
The patient was brought back to the interventional suite the following day for definitive treatment. Using similar technique, the SMA branch was catheterized and the micro-catheter was placed distal to the aneurysm after passing through the previously placed stent. In order to pass the microcatheter distal to the aneurysm, it was necessary to enter it with the guidewire and microcatheter and loop the system inside the aneurysm before passing out well beyond its neck, in order to deploy the flow diverter. Subsequently, a 3 mm×35 mm Pipeline Flex flow diverter (Medtronic, Irvine, CA) was delivered to the tip of the microcatheter but not deployed. The redundant loop of microcatheter was then retracted, followed by careful deployment of the Pipeline flow diverter across the pseudoaneurysm neck in the desired location. Control angiography demonstrated optimal device position but persistent vigorous filling of the pseudoaneurysm. A second Pipeline device (3 mm×20 mm) was then deployed inside the first device in a “telescoping” fashion, with demonstrated excellent stasis of contrast inside the aneurysm (
Fig. 4). The patient tolerated the procedure well. He was placed on dual antiplatelet therapy. Follow-up CT angiography performed twenty months post-procedure demonstrated complete SAA exclusion with maintained patency of the Pipeline Embolization Device and inferior segmental artery. At 1-year follow-up the patient is asymptomatic and disease-free on imaging.
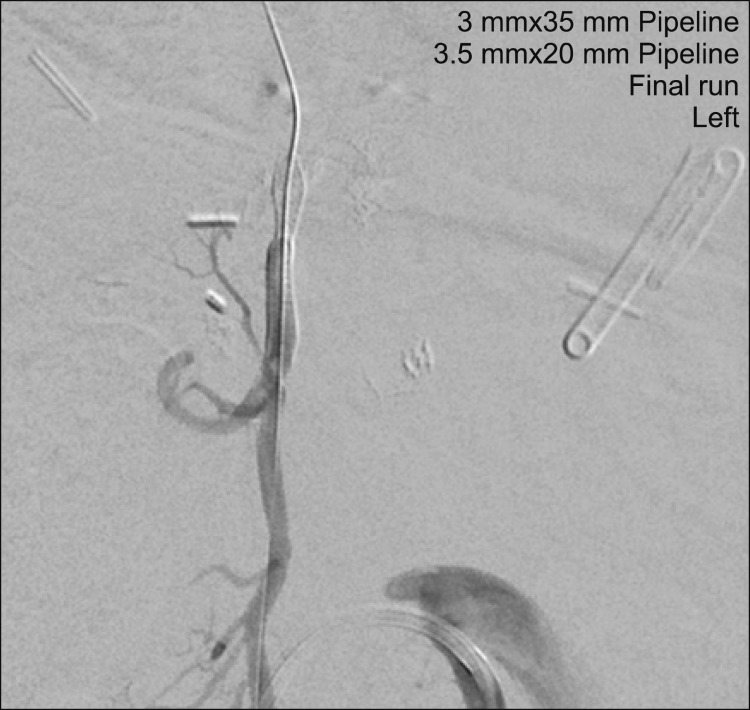 | Fig. 4Control SMA angiography post deployment of flow-diverting devices. Control SMA angiography after positioning of the first device (3 mm×35 mm Pipeline Flex flow diverter) demonstrated optimal device position but persistent vigorous filling of the pseudoaneurysm. Deployment of a second Pipeline device (3 mm×20 mm) inside the first device in a “telescoping” fashion demonstrated excellent stasis of contrast inside the aneurysm.
|
Go to :

DISCUSSION
Even though rather uncommon, pancreatic pseudoaneurysms can be life threatening. They develop when pancreatic secretions result in autodigestion of the adjacent arterial walls. Pancreatitis is the major pancreatic pseudoaneurysm formation cause, with an incidence as high as 10%. Pancreatic pseudoaneurysms may also develop after biliopancreatic resections or after pancreas transplantation.
1 The focal inflammation with or without sepsis triggered by the presence of an anastomotic leak may result in vessel erosion with pseudoaneurysm formation and delayed rupture and bleed. The splenic artery is the most frequent site of visceral artery pseudoaneurysms, followed by the hepatic artery.
2
Based on the originating artery type, presence of gastrointestinal tract communication and pancreatic juice exposure, Pang et al.
3 developed a management-based classification system for peripancreatic pseudoaneurysms.
Rupture carries a 13–40% mortality risk and is almost universally fatal if left untreated. Therefore, timely identification and management is of essence. Owing to interventional radiology advances, the standard of care has shifted from surgical intervention to endovascular treatment.
4 Digital subtraction facilitates high resolution mapping of the peripancreatic vessels and synchronous percutaneous intervention. Pre-operative angiography allows for identification of the offending vessels and the opportunity to gain temporary control of the bleed.
5
Pseudoaneurysm treatment options include endovascular coil embolization, placement of covered stent
6, percutaneous thrombin injection or open surgical. Embolization is less invasive than surgical exploration and endovascular coils have been used extensively for treating visceral aneurysms.
278 It is well tolerated by the patients and quick to perform. Should surgical exploration be still indicated, angioembolization would allow surgery in optimized conditions.
The pseudoaneurysm embolization carries a reported 67–100% success rate.
79 Its failure is usually due to inability to identify and selectively catheterize the offending vessel or misplacement of the embolization material. Possible complications are rebleeding, pseudoaneurysm rupture, arterial perforation, aortic thrombosis and bowel ischemia.
The Pipeline™ Flex Embolization device (Medtronic, Irvine, CA) is indicated for the endovascular treatment of large or giant wide-necked intracranial aneurysms. Its safety and efficacy in the treatment of complex intracranial aneurysms of the internal carotid artery has been assessed by the PUFs (Pipeline™ Embolization Device for Uncoilable or Failed Aneurysms), which reported 95% occlusion at 5 years with 0% complication and 0% recurrence rates.
10 Application of flow diversion Pipeline™ technology further extended in the management of posterior circulation and extracranial vertebral artery aneurysms, which would be impossible to manage with clipping or coil embolization.
1112
Despite the extensive application of Pipeline flow diversion in the management of intracranial or extracranial neurosurgical cases, its application in the abdominal visceral vasculature is extremely limited, with only a few reported applications, specifically in renal, splenic and post-transplant hepatic artery pseudoaneurysms.
1314, 15 Adrachtas et al.
13 reported the successful use of Pipeline Embolization Device (Medtronic, Irvine, CA) for the endovascular treatment of a 40-year-old patient with a complex renal artery aneurysm. On 3-year follow-up, vascular imaging showed complete occlusion of the aneurysm and normal renal function.
13 Abraham et al. reported the successful management of a symptomatic 4.1 cm splenic artery aneurysm (SAA) with selective two-staged segmental splenic artery embolization with the deployment of Pipeline Embolization Device (ev3 Endovascular Inc, Plymouth, Minnesota), which allowed exclusion of the aneurysm at its neck while maintaining splenic perfusion.
14
In our case, the application of PipelineTM Flex (Medtronic, Irvine, CA) flow diversion technology allowed the preservation of patency of the main arterial supply to the gastric conduit on a post-esophagectomy patient; loss of the right gastroepiploic artery in that case would had been otherwise catastrophic.
Use of flow diversion technology to occlude a bleeding pseudoaneurysm preserved the main arterial supply to the gastric conduit, in a case where there was no other surgical.
Go to :







 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download