1. Case 1
A 31-year-old female patient was referred by her orthodontist to Asan Medical Center in Seoul, Korea for treatment of facial asymmetry and prognathism. The patient had never been diagnosed with a medical problem and had no previous history of fracture. Clinical examination revealed that the patient was slightly short in height (153.4 cm) and moderately overweight (62.4 kg, body mass index [BMI] 26.52 kg/m
2). Examination of her facial features revealed a long lower face with vertical maxillary excess and mandibular asymmetry with occlusal canting of the maxilla. In addition, she had upper lip protrusion and lip incompetence.(
Fig. 1,
2) The intraoral findings included Class III malocclusion with an open bite. She had normal tooth structure and received proper dental care. Therefore, her periodontal health and oral hygiene were good.
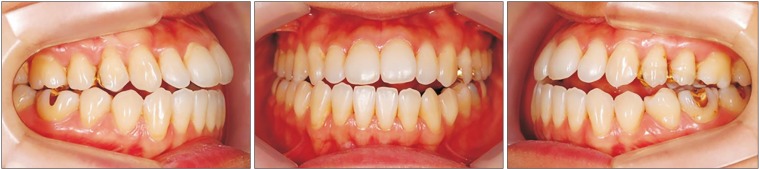 | Fig. 1Preoperative intraoral photographs of Case 1 patient.
|
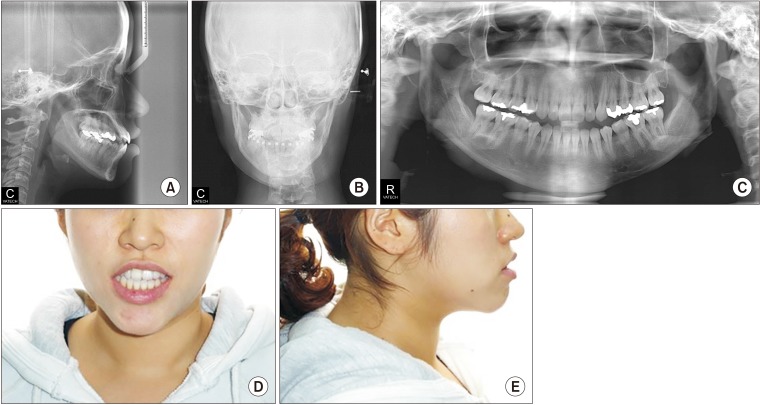 | Fig. 2Preoperative cephalometric (A, B), panoramic (C), and facial views (D, E) of Case 1 patient.
|
After the initial interview, the patient was referred to an orthodontist for leveling and alignment of the teeth, and treatment continued for approximately one year. After the presurgical orthodontic treatment, a clinical evaluation was repeated, and cephalometric radiographs and face bow recordings were obtained for final surgical planning 4 weeks prior to the surgery.
In addition, a work-up for general anesthesia and surgery was performed, and the preoperative hematologic examination, electrocardiogram and chest radiograph were unremarkable.
The surgery included bimaxillary procedures. Le Fort I osteotomy with midline correction, canting correction, posterior impaction, and setback movement of the maxilla were performed via rigid skeletal fixation. Bilateral sagittal split ramus osteotomies were performed with a setback movement via the hybrid fixation technique (with one miniplate and one additional bicortical screw). On the left side, a greenstick fracture occurred in the proximal segment. However, we could appropriately position the proximal and distal segments as planned, and so two additional bicortical screws were used to fix the fractured proximal segment. Two closed suction drains were inserted into the mandibular surgical site. The operation was completed without major bleeding, and the estimated blood loss during the surgery was 200 mL.
Abnormal edema and ecchymosis were observed from the second postoperative day.(
Fig. 3) At first, an improperly functioning closed suction drain was suspected, but the ecchymosis and edema appeared to be bilateral, decreasing the likelihood of drain malfunction. On the second postoperative day, drain removal and maxilla-mandibular fixation (MMF) with elastic ring were routinely performed. Normally, an orthognathic surgical patient is hospitalized for 2 nights and 3 days after surgery, but in this case, the discharge was delayed due to the appearance of abnormal ecchymosis and edema. On the fifth postoperative day, nasal bleeding and transient blood pressure lowering were noted, but the problems resolved after nasal packing and administration of intravenous fluids. At 6 days postoperatively, the patient was stable, and she was discharged from the hospital.
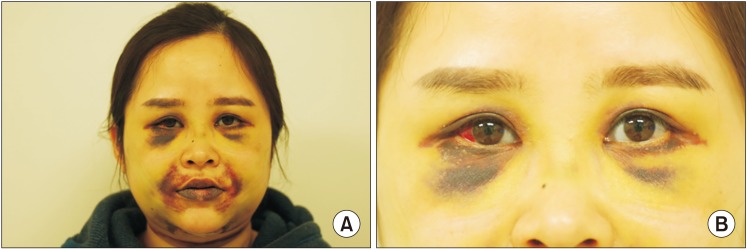 | Fig. 3Photographs on postoperative 9 days of Case 1 patient; Ecchymosis and abnormal edema (A), subconjunctival hemorrhage, blue sclera (B).
|
MMF was routinely stopped 2 weeks after surgery, and training elastics were applied. No other complications were observed. However, the ecchymosis persisted for 6 weeks, and although it dissipated slowly and spontaneously, no specific treatment was needed for its resolution. The follow-up examination at postoperative 3 months showed uneventful healing.
During the search for the cause of unexpected postoperative bleeding, she recounted multiple fractures of the lower limbs during childhood, and blue sclera was noticed. Furthermore, her familial history included multiple bone fractures in her mother, grandmother, and cousin. Therefore, OI type I was suspected, and it was inferred that the patient's complications were ultimately caused by bleeding due to vessel fragility from OI.
The postsurgical orthodontic treatment began one month after surgery. The molar and canine relation became Class I, and the open bite was greatly improved. After approximately eight months, debonding was done. The long lower face with vertical maxillary excess and mandibular asymmetry with occlusal canting of the maxilla were greatly improved. The upper lip protrusion and lip incompetence were also improved.(
Fig. 4,
5)
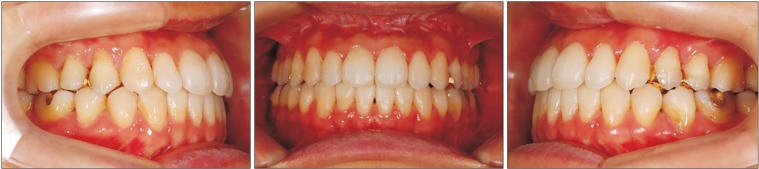 | Fig. 4Intraoral photographs on debonding of Case 1 patient.
|
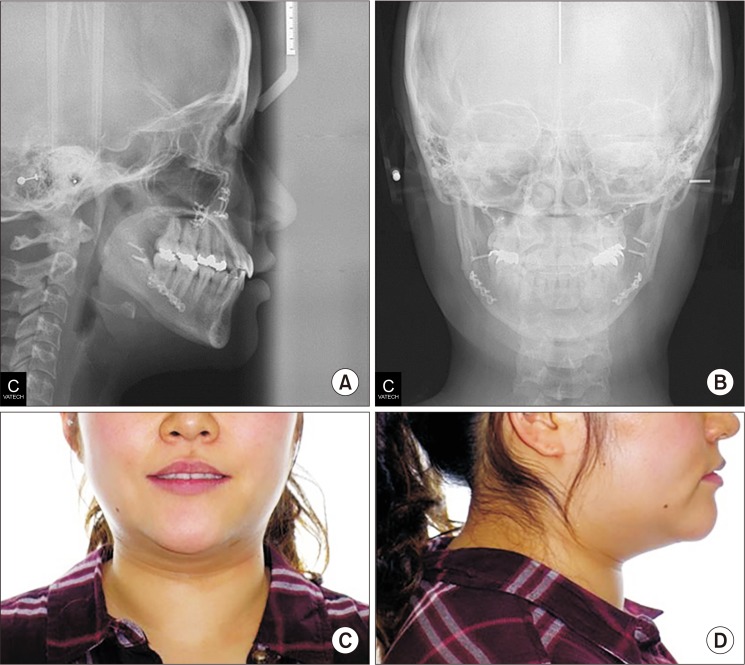 | Fig. 5Cephalometric (A, B) and facial views (C, D) on debonding of Case 1 patient.
|
2. Case 2
A 33-year-old female patient was referred by her orthodontist to Asan Medical Center in Seoul, Korea for the treatment of facial asymmetry and chin protrusion. She was the older sister of the patient from Case 1, and she appeared similar to her younger sister.
The patient also had never been previously diagnosed with a medical problem, but she did have a history of surgery for a left leg bone fracture in her teenage years. The general examination revealed that the patient was slightly short in height (155.0 cm) and slightly overweight (52.8 kg, BMI 21.98 kg/m2), similar to her younger sister.
As for her facial features, the patient had a facial appearance similar to her sister's, and she had the following similar problems. She had a long lower face with vertical maxillary excess and mandibular asymmetry with occlusal canting and yawing of the maxilla. Upper lip protrusion and chin protrusion were also apparent.(
Fig. 6,
7) Intraoral findings included Class III malocclusion with a shallow overbite. Likewise, she had normal teeth structure, and she also had good periodontal health and oral hygiene due to proper dental care.
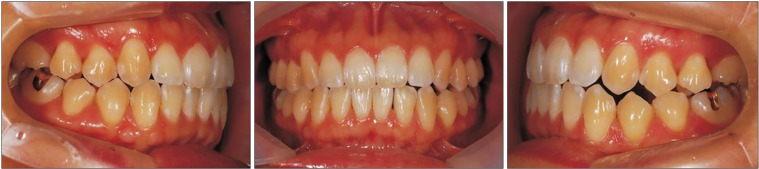 | Fig. 6Preoperative intraoral photographs of Case 2 patient.
|
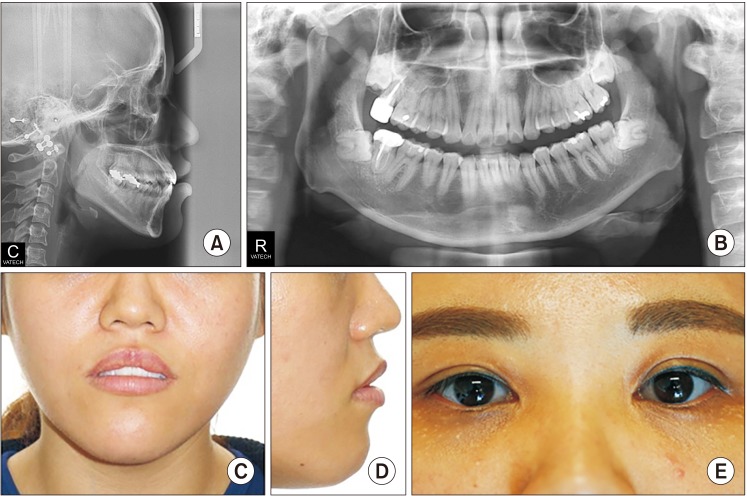 | Fig. 7Preoperative cephalometric (A), panoramic (B), and facial views (C, D) of Case 2 patient and her blue sclera (E) can be seen.
|
She was referred to the same orthodontist as her sister for one year of orthodontic treatment before surgery. After the presurgical orthodontic treatment, the clinical evaluation was repeated, and cephalometric radiographs and face bow recordings were obtained for final surgical planning 4 weeks prior to the surgery.
In addition, a work-up for general anesthesia and surgery were performed, and all results were normal. However, during the patient's surgical preparation, OI was critically considered due to her family history and her younger sister's operative complications.
The possibility of hemorrhage due to OI was explained to the patient prior to surgery. Furthermore, we noted that the operation could be stopped and changed to a two-step procedure if massive bleeding occurred during surgery.
The surgical plan was similar to that of the patient from Case 1 as the sisters had similar craniofacial relationships. The surgery included bimaxillary procedures. Le Fort I osteotomy with midline correction, canting correction, posterior impaction, and setback movement of the maxilla were performed using rigid skeletal fixation. Bilateral sagittal split ramus osteotomies were performed with a setback movement using the hybrid fixation technique (one miniplate and one additional bicortical screw). On the left side, a greenstick fracture occurred in the proximal segment, as in the younger sister's case, but we could appropriately position the proximal and distal segments as planned, and so two additional bicortical screws were used to fix the fractured proximal segment. Two closed suction drains were inserted into the mandibular surgical site. The operation was completed without major bleeding, and the blood loss during the surgery was estimated to 200 mL.
The patient's recovery process proceeded routinely, and there was no remarkable finding. Daily dressing was done, and on the second postoperative day, drain removal and MMF with elastic ring were performed. On the third postoperative day, the patient was discharged without any abnormal complications.
MMF was stopped 2 weeks after surgery, and training elastics were applied. No other complications were observed. The follow-up examination at postoperative 6 months showed uneventful healing.
The postsurgical orthodontic treatment began one month after surgery. After Class I molar, canine relationship, normal overbite and overjet were achieved, debonding was done. The patient's long lower face with vertical maxillary excess and mandibular asymmetry with occlusal canting and yawing of the maxilla were greatly improved. Upper lip protrusion and chin protrusion were also improved.(
Fig. 8,
9) No specific problems during orthodontic treatment were encountered.
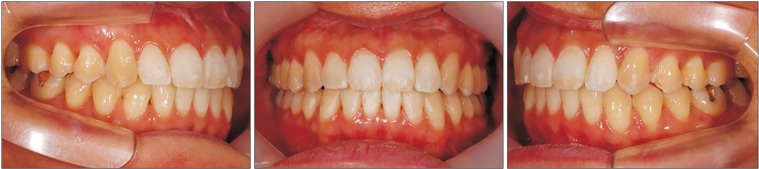 | Fig. 8Intraoral photographs on debonding of Case 2 patient.
|
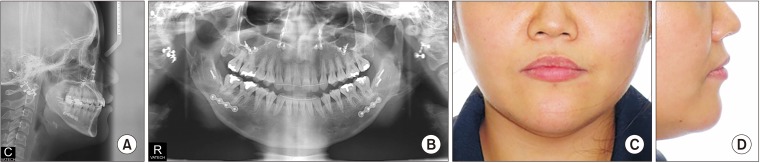 | Fig. 9Cephalometric (A), panoramic (B), and facial views (C, D) of Case 2 patient.
|