1. Antony PG, Sebastian A, Varghese KG, Sobhana CR, Mohan S, Soumithran CS, et al. Neurosensory evaluation of inferior alveolar nerve after bilateral sagittal split ramus osteotomy of mandible. J Oral Biol Craniofac Res. 2017; 7:81–88. PMID:
28706780.

2. Aizenbud D, Ciceu C, Hazan-Molina H, Abu-El-Naaj I. Relationship between inferior alveolar nerve imaging and neurosensory impairment following bilateral sagittal split osteotomy in skeletal class III cases with mandibular prognathism. Int J Oral Maxillofac Surg. 2012; 41:461–468. PMID:
22115977.

3. Tamás F. Position of the mandibular canal. Int J Oral Maxillofac Surg. 1987; 16:65–69. PMID:
3104497.

4. Ylikontiola L, Kinnunen J, Oikarinen K. Factors affecting neurosensory disturbance after mandibular bilateral sagittal split osteotomy. J Oral Maxillofac Surg. 2000; 58:1234–1239. PMID:
11078134.

5. Brusati R, Fiamminghi L, Sesenna E, Gazzotti A. Functional disturbances of the inferior alveolar nerve after sagittal osteotomy of the mandibular ramus: operating technique for prevention. J Maxillofac Surg. 1981; 9:123–125. PMID:
6943243.

6. Ylikontiola L, Moberg K, Huumonen S, Soikkonen K, Oikarinen K. Comparison of three radiographic methods used to locate the mandibular canal in the buccolingual direction before bilateral sagittal split osteotomy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002; 93:736–742. PMID:
12142882.

7. Westermark A, Bystedt H, von Konow L. Inferior alveolar nerve function after mandibular osteotomies. Br J Oral Maxillofac Surg. 1998; 36:425–428. PMID:
9881783.

8. Hasani A, Ahmadi Moshtaghin F, Roohi P, Rakhshan V. Diagnostic value of cone beam computed tomography and panoramic radiography in predicting mandibular nerve exposure during third molar surgery. Int J Oral Maxillofac Surg. 2017; 46:230–235. PMID:
27810140.

9. Neves FS, Souza TC, Almeida SM, Haiter-Neto F, Freitas DQ, Bóscolo FN. Correlation of panoramic radiography and cone beam CT findings in the assessment of the relationship between impacted mandibular third molars and the mandibular canal. Dentomaxillofac Radiol. 2012; 41:553–557. PMID:
22282507.

10. Arora A, Patil BA, Sodhi A. Validity of the vertical tube-shift method in determining the relationship between the mandibular third molar roots and the inferior alveolar nerve canal. J Korean Assoc Oral Maxillofac Surg. 2015; 41:66–73. PMID:
25922817.

11. Szalma J, Lempel E, Jeges S, Szabó G, Olasz L. The prognostic value of panoramic radiography of inferior alveolar nerve damage after mandibular third molar removal: retrospective study of 400 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010; 109:294–302. PMID:
19846324.

12. Kositbowornchai S, Densiri-aksorn W, Piumthanaroj P. Ability of two radiographic methods to identify the closeness between the mandibular third molar root and the inferior alveolar canal: a pilot study. Dentomaxillofac Radiol. 2010; 39:79–84. PMID:
20100918.

13. Tantanapornkul W, Okochi K, Bhakdinaronk A, Ohbayashi N, Kurabayashi T. Correlation of darkening of impacted mandibular third molar root on digital panoramic images with cone beam computed tomography findings. Dentomaxillofac Radiol. 2009; 38:11–16. PMID:
19114418.

14. Nakamori K, Tomihara K, Noguchi M. Clinical significance of computed tomography assessment for third molar surgery. World J Radiol. 2014; 6:417–423. PMID:
25071882.

15. Obwegeser HL. Orthognathic surgery and a tale of how three procedures came to be: a letter to the next generations of surgeons. Clin Plast Surg. 2007; 34:331–355. PMID:
17692696.

16. Westermark A, Bystedt H, von Konow L. Patients' evaluation of the final result of sagittal split osteotomy: is it influenced by impaired sensitivity of the lower lip and chin. Int J Adult Orthodon Orthognath Surg. 1999; 14:135–139. PMID:
10686836.
17. Yamamoto R, Nakamura A, Ohno K, Michi KI. Relationship of the mandibular canal to the lateral cortex of the mandibular ramus as a factor in the development of neurosensory disturbance after bilateral sagittal split osteotomy. J Oral Maxillofac Surg. 2002; 60:490–495. PMID:
11988921.

18. Rajchel J, Ellis E 3rd, Fonseca RJ. The anatomical location of the mandibular canal: its relationship to the sagittal ramus osteotomy. Int J Adult Orthodon Orthognath Surg. 1986; 1:37–47. PMID:
3457874.
19. Yoshioka I, Tanaka T, Khanal A, Habu M, Kito S, Kodama M, et al. Relationship between inferior alveolar nerve canal position at mandibular second molar in patients with prognathism and possible occurrence of neurosensory disturbance after sagittal split ramus osteotomy. J Oral Maxillofac Surg. 2010; 68:3022–3027. PMID:
20739116.

20. De Vos W, Casselman J, Swennen GR. Cone-beam computerized tomography (CBCT) imaging of the oral and maxillofacial region: a systematic review of the literature. Int J Oral Maxillofac Surg. 2009; 38:609–625. PMID:
19464146.

21. Hashiba Y, Ueki K, Marukawa K, Shimada M, Yoshida K, Shimizu C, et al. A comparison of lower lip hypoesthesia measured by trigeminal somatosensory-evoked potential between different types of mandibular osteotomies and fixation. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007; 104:177–185. PMID:
17448708.

22. Anderson LC, Kosinski TF, Mentag PJ. A review of the intraosseous course of the nerves of the mandible. J Oral Implantol. 1991; 17:394–403. PMID:
1813647.
23. Gowgiel JM. The position and course of the mandibular canal. J Oral Implantol. 1992; 18:383–385. PMID:
1298823.
24. Ozturk A, Potluri A, Vieira AR. Position and course of the mandibular canal in skulls. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012; 113:453–458. PMID:
22676925.

25. Colella G, Cannavale R, Vicidomini A, Lanza A. Neurosensory disturbance of the inferior alveolar nerve after bilateral sagittal split osteotomy: a systematic review. J Oral Maxillofac Surg. 2007; 65:1707–1715. PMID:
17719387.

26. Al-Bishri A, Barghash Z, Rosenquist J, Sunzel B. Neurosensory disturbance after sagittal split and intraoral vertical ramus osteotomy: as reported in questionnaires and patients' records. Int J Oral Maxillofac Surg. 2005; 34:247–251. PMID:
15741031.

27. Cunningham LL, Tiner BD, Clark GM, Bays RA, Keeling SD, Rugh JD. A comparison of questionnaire versus monofilament assessment of neurosensory deficit. J Oral Maxillofac Surg. 1996; 54:454–459. PMID:
8600262.

28. Chen N, Neal CE, Lingenbrink P, Bloomquist D, Kiyak HA. Neurosensory changes following orthognathic surgery. Int J Adult Orthodon Orthognath Surg. 1999; 14:259–267. PMID:
10895640.
29. Bell WH. Modern practice in orthognathic and reconstructive surgery. Philadelphia: Saunders;1992.
30. Manor Y, Blinder D, Taicher S. Sequence of treatment in mandibular prognathism patients. Cranio. 2006; 24:95–97. PMID:
16711270.

31. Romeo U, Del Vecchio A, Palaia G, Tenore G, Visca P, Maggiore C. Bone damage induced by different cutting instruments--an in vitro study. Braz Dent J. 2009; 20:162–168. PMID:
19738951.
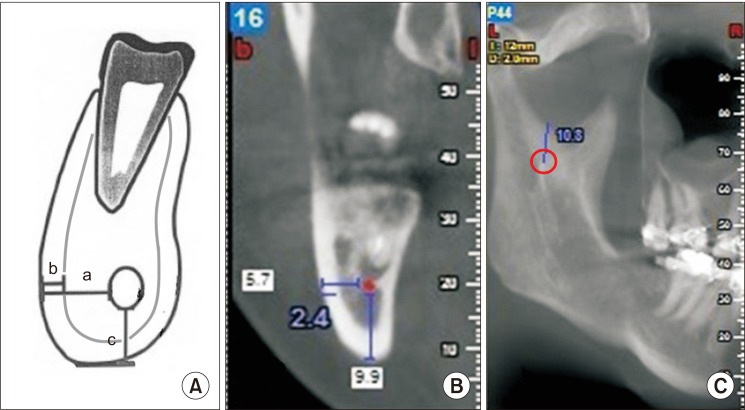




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download