Abstract
Hepatocellular carcinoma, a disease of the developing world, is known to present with extrahepatic metastases. Most common site being the lungs, it is not uncommon for metastases to present at unusual sites like the rectum, spleen and the diaphragm, among others. Metastases to the oral cavity is rare, with the most common primaries being lung, breast and the kidney. Metastases of a hepatocellular carcinoma to the oral cavity is a rare entity with extremely limited data in literature. We present one such unique case of oral cavity metastases from a hepatocellular carcinoma who presented to the Division of Head and Neck Oncology services of our hospital with a large oral cavity lesion, on subsequent workup of which, a hepatocellular carcinoma was identified. Awareness of this possibility can aid in accurate diagnosis and early management of a condition associated with an advanced stage at presentation and poorer prognosis.
Hepatocellular carcinoma (HCC) is the most common primary liver malignancy comprising 75–85% of all primary liver tumours. Majority of cases occur in the developing world, especially Southeast Asia and sub-Saharan Africa, where the major etiologic factor is exposure to hepatitis B virus (HBV) and hepatitis C virus (HCV). Other etiologic factors that are important in developed countries are cirrhosis due to any cause, alcohol abuse, obesity, and hemochromatosis. In general these tumors have a poor prognosis, compounded by the background liver disease in majority of the patients. Extra-hepatic metastasis of HCC occurs in about 30-50% of patients, and it depends on the stage.123 The commonest sites of extra-hepatic metastases of HCC are the lungs,123 followed by intra-abdominal lymph nodes, bones and adrenal glands. Unusual sites of metastases include rectum, spleen, diaphragm, esophagus, pancreas and the urinary bladder. Usually extra-hepatic metastases corrleate with advancing intra-hepatic tumor stage but occasionally the extrahepatic metastatic site may be the initial presenting feature particularly when the tumor metastasizes to an unusual site. We report a rare case of extra-hepatic metastasis from an HCC to the oral cavity presenting with a large buccal mucosal lesion.
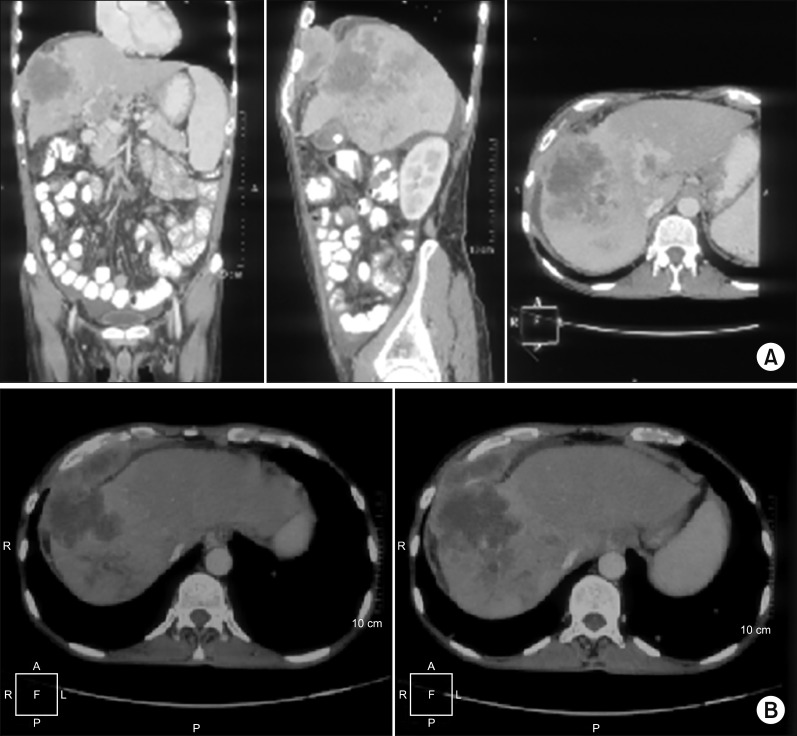
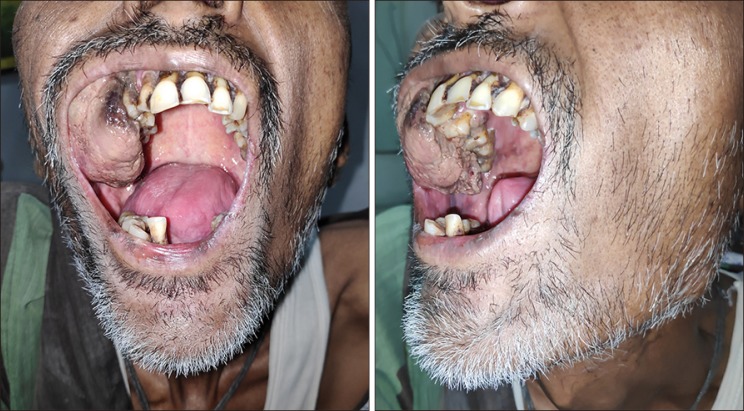
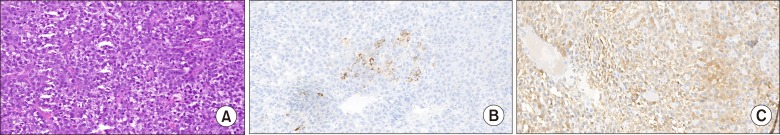
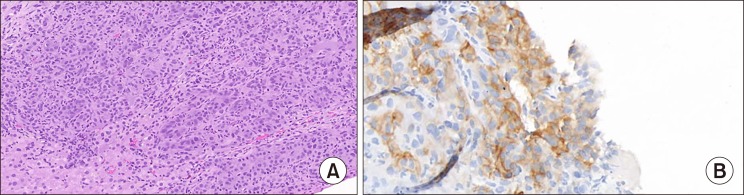
49-year-old gentleman presented to the outpatient department of Head and Neck Oncology services of our institution with complaints of a large lesion in the oral cavity for a duration of one month. The patient also complained of dyspepsia, generalized weakness, malaise and loss of appetite. On examination, the general condition of the patient was poor in view of cancer related cachexia. He had a soft tissue lesion of the upper alveolus extending from the right upper canine to the right superior retro-molar trigone involving the entire upper alveolus and extending into the upper gingivobuccal sulcus (Fig. 1). The mass bled to touch but was non tender. Medially, it extended onto the hard palate for about half a centimetre. On abdominal examination, a mass was palpable in the right hypochondrium extending to the epigastrium. Rest of the abdomen was soft and non-tender. On further evaluation with a contrast enhanced computed tomography of the thorax, abdomen and pelvis, a 7×7×7 cm hypodense mass was noted in the segment VII and VIII of liver, which showed enhancement on arterial phase with washout on venous phase (Fig. 2). The mass was infiltrating the hepatic capsule with a peritoneal deposit abutting the diaphragm and muscles of the chest wall. Enhancing tumour thrombus was noted in the distended main portal vein and its branches. On upper gastrointestinal endoscopy, no varices were noted. Serum alpha fetoprotein was 2440 ng/ml. Liver function tests were normal. He was detected to be positive for Hepatitis B surface antigen. Blocks of the alveolar growth biopsy done elsewhere were reviewed at our hospital and was found to be a metastatic deposit of hepatocellular carcinoma positive for HepPar 1, arginase and focally positive for Glypican 3 whereas negative for CK7, p63, and DOG1 on immunohistochemistry (IHC) (Fig. 3). An ultrasound guided biopsy of the liver lesion was suggestive of hepatocellular carcinoma, positive for Glypican 3 on IHC (Fig. 4).
In view of advanced metastatic disease and poor performance status the patient was declared to receive the best possible supportive care. Episodes of bleeding from the alveolar lesion were managed conservatively.
Metastatic malignant tumours of the oral cavity are rare. The most common primaries are lung, breast, and renal cell carcinomas.45 The metastasis to oral cavity usually relates to extensive tumor spreading and occurs relatively late. Besides its low incidence rate, the easily ignored clinical symptoms and signs also contribute to the rare presentation of pathologically confirmed oral cavity metastasis in patients with advanced HCC.
Pathogenesis of such metastasis is thought to be associated with oral inflammation that possibly attracts migration and adhesion of cancer cells to the oral cavity mucosa, in which some inflammatory molecules might play key roles.6 Few authors have proposed that the localized slowing of blood flow contribute to oral cavity metastasis by favoring the fall-out of malignant cells.78 The hematogenous route through portal vessels is the preferred mode for oral metastasis; however, metastatic pulmonary tumors are not found in some cases as expected.9 In such instances, the pulmonary circulation is bypassed through a candidate pathway of valve-less vertebral venous plexus (Batson's plexus) which has been the proposed explanation, and remains to be anatomically and experimentally verified.810 Moreover, the regional lymphatic vessels are a possible route for oral cavity metastasis by HCC.11
Up until now, the survival of gingival metastasis by HCC is not very clear because of the rarity of such cases, which are usually published without a detailed and systematic description. According to limited series with survival data in English literature, the median time of overall and truncated survival for HCC patients with gingival metastasis is about 11.5 months (range, 2 to 92 months) and 3.5 months (range, 1 to 15 months), respectively.6
The diagnosis of a metastatic lesion in the oral cavity is a challenge to clinicians due to the lack of pathognomonic signs and symptoms. Oral metastases usually occur in the advanced stages of cancers, and the interval between appearance and death is usually short. Awareness regarding the possibility of oral cavity metastases in HCC helps aid the diagnosis especially in patients where the primary tumour has not yet been identified. Biopsy of the oral lesion with IHC suggestive of HCC is usually diagnostic. Such patients presenting with oral metastases usually have a poor prognosis with majority already harboring widespread metastatic disease on further detailed evaluation. In such cases, palliation of symptoms remains the only modality of treatment.
References
1. Sawabe M, Nakamura T, Kanno J, Kasuga T. Analysis of morphological factors of hepatocellular carcinoma in 98 autopsy cases with respect to pulmonary metastasis. Acta Pathol Jpn. 1987; 37:1389–1404. PMID: 2825465.

2. Katyal S, Oliver JH 3rd, Peterson MS, Ferris JV, Carr BS, Baron RL. Extrahepatic metastases of hepatocellular carcinoma. Radiology. 2000; 216:698–703. PMID: 10966697.

3. Natsuizaka M, Omura T, Akaike T, Kuwata Y, Yamazaki K, Sato T, et al. Clinical features of hepatocellular carcinoma with extrahepatic metastases. J Gastroenterol Hepatol. 2005; 20:1781–1787. PMID: 16246200.

4. Will TA, Agarwal N, Petruzzelli GJ. Oral cavity metastasis of renal cell carcinoma: a case report. J Med Case Rep. 2008; 2:313. PMID: 18823541.

5. Makos CP, Psomaderis K. A literature review in renal carcinoma metastasis to the oral mucosa and a new report of an epulis-like metastasis. J Oral Maxillofac Surg. 2009; 67:653–660. PMID: 19231796.

6. Hirshberg A, Leibovich P, Buchner A. Metastases to the oral mucosa: analysis of 157 cases. J Oral Pathol Med. 1993; 22:385–390. PMID: 8301602.

8. Appenzeller J, Weitzner S, Long GW. Hepatocellular carcinoma metastatic to the mandible: report of case and review of literature. J Oral Surg. 1971; 29:668–671. PMID: 4328199.
9. Ramón Ramirez J, Seoane J, Montero J, Esparza Gómez GC, Cerero R. Isolated gingival metastasis from hepatocellular carcinoma mimicking a pyogenic granuloma. J Clin Periodontol. 2003; 30:926–929. PMID: 14710773.
10. Batson OV. The function of the vertebral veins and their role in the spread of metastases. Ann Surg. 1940; 112:138–149. PMID: 17857618.

11. Lund BA, Soule EH, Moertel CG. Hepatocellular carcinoma with metastasis to gingival mucosa: report of case. J Oral Surg. 1970; 28:604–607. PMID: 4317000.
Fig. 2
(A) CECT abdomen & pelvis; coronal, sagittal and axial sections showing the liver primary with diaphragm & chest wall abutment. (B) CECT abdomen & pelvis axial section showing the liver primary with diaphragm & chest wall abutment.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download