Abstract
Figures and Tables
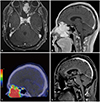 | Fig. 1Preoperative magnetic resonance imaging (MRI) and 68Ga-DOTA-congugated octreotide positron emission tomography (PET)/computed tomography (CT) scan. Axial and sagittal views of T1-weighted post-contrast MRI show a heterogeneously enhanced mass in the nasal cavity, sphenoid sinus, and frontal base (A and B). 68Ga-DOTA-congugated octreotide PET/CT scan reveals high somatostatin uptake in the tumor, which is suggestive of a neuroendocrine tumor (C). Twelve-month postoperative MRI shows no evidence of a remnant or recurrent tumor (D). |
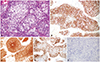 | Fig. 2High-power view of H&E staining shows small- to medium-sized neoplastic cells with lobulation. Necrosis and mitosis are not seen. Immunohistochemistry (IHC) staining for various cell markers revealed positive staining for adrenocorticotropic hormone (ACTH), neuron-specific enolase (NSE), and chromogranin A and negative staining for T-Pit. (A) H&E ×200, (B) ACTH-IHC ×200, (C) NSE-IHC ×200, (D) Chromogranin A-IHC ×200, and (E) T-Pit-IHC ×200. |
ACKNOWLEDGEMENTS
Notes
AUTHOR CONTRIBUTIONS
Conceptualization: Eui Hyun Kim.
Data curation: Young Soo Chung, Minkyun Na, and Se Hoon Kim.
Formal analysis: Eui Hyun Kim and Young Soo Chung.
Funding acquisition: Eui Hyun Kim.
Methodology: Eui Hyun Kim and Young Soo Chung.
Supervision: Cheol Ryong Ku and Eui Hyun Kim.
Validation: Eui Hyun Kim.
Visualization: Eui Hyun Kim and Young Soo Chung.
Writing—original draft: Young Soo Chung.
Writing—review & editing: Cheol Ryong Ku and Eui Hyun Kim.
Approval of final manuscript: All authors.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download