Abstract
Purpose
Materials and Methods
Results
Figures and Tables
 | Fig. 1Trends in reported AEs after vaccination with PPSV and PCV compared to reported AEs after vaccination with all vaccines from KIDS database in 2005–2016. AE, adverse event; PPSV, pneumococcal polysaccharide vaccine; PCV, pneumococcal conjugate vaccine; KIDS, Korea Institute of Drug Safety and Risk Management. |
Table 1
Demographic Characteristics of PPSV and PCV Recipients Whose Adverse Events Were Reported to the Korea Adverse Event Reporting System (KAERS), 2005–2016
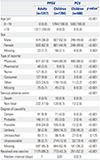
Table 3
Signals of Adverse Events Related to PPSVs in Adults Aged ≥19 Years Detected by Data Mining and the Presence of Information on Vaccine Label, 2005–2016

Table 4
Signals of Adverse Events Related to PPSVs for Children Aged 0–18 Years Detected by Data Mining and the Presence of Information on Vaccine Label, 2005–2016

PPSVs, pneumococcal polysaccharide vaccines; PRR, proportional reporting ratio; ROR, reporting odds ratio; IC 95% LCI, information component lower limit of 95% confidence interval; SGOT, serum glutamic oxaloacetic transaminase; SGPT, serum glutamic pyruvic transaminase.
*Y: AE was listed on the vaccine label, N: AE was not listed on vaccine label.
Table 5
Signals for Adverse Events of PCVs in Children Aged 0–18 Years Detected by Data Mining and the Presence of Information on Vaccine Label, 2005–2016

ACKNOWLEDGEMENTS
Notes
AUTHOR CONTRIBUTIONS
Conceptualization: Kwan Soo Kim, Min Soo Park, and Ju-Young Shin.
Data curation: Kwan Soo Kim and In-Sun Oh.
Formal analysis: In-Sun Oh.
Supervision: Min Soo Park and Ju-Young Shin
Writing—original draft: Kwan Soo Kim.
Writing—review & editing: Kwan Soo Kim, Hyun Jeong Kim, Inmyung Song, Min Soo Park, and Ju-Young Shin.
Approval of final manuscript: all authors.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download