Abstract
Purpose
Materials and Methods
Results
Figures and Tables
 | Fig. 1Muscle activity of the upper limb on surface EMG during rest and five trials of Task 1 with the opposite hand in two children with unilateral CP. (A) Visible AR (+): muscle activities of the elbow extensor, wrist flexor, and wrist extensor in the more-affected side increased during tasks with the less-affected hand, compared to baseline activities at rest. (B) Visible AR (−): muscle activities of the upper limb in the less-affected side during tasks with the more-affected hand did not increase, compared to baseline activities at rest. CP, cerebral palsy; AR, associated reaction. |
Table 1
Characteristics of the Participants
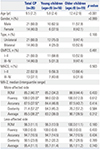
CP, cerebral palsy; GMFCS, Gross Motor Function Classification System; MACS, Manual Ability Classification System; MA-2, Melbourne Assessment 2; ROM, range of motion.
Values are presented as a median (interquartile range) unless otherwise noticed.
*p<0.001, compared to the less-affected side in the total CP group and based on Wilcoxon signed rank test.
Table 2
Comparison of Visible AR Frequencies and AR Scores
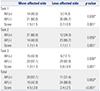
AR, associated reaction.
Values are presented as a number (%) or mean±standard deviation. Task 1, opening and clenching the fist; Task 2, a finger opposition task; Task 3, tapping fingers on the table surface.
*Comparison of AR frequency with side based on Fisher's exact test, †Comparison of AR score with side based on Wilcoxon signed rank test.
Table 3
Comparison of AR Frequencies between Good and Less Functioning Groups and Comparison of Upper Arm Function between Groups with and without AR

AR, associated reaction; GMFCS, Gross Motor Function Classification System; MACS, Manual Ability Classification System; MA-2, Melbourne Assessment 2; ROM, range of motion.
Values are presented as a number (%) or mean±standard deviation.
*p<0.05 of GMFCS and MACS based on Fisher exact test, †p<0.05 of MA-2 based on Mann-Whitney U-test.
Table 5
Subgroup Analysis according to Age and Bimanual Ability
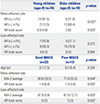
AR, associated reaction; MACS, Manual Ability Classification System; MA-2, Melbourne Assessment 2.
Values are presented as a median (interquartile range) unless otherwise noticed.
*p<0.05 of AR frequency based on Fisher's exact test, †p<0.05 of age, MA-2 and AR total score based on Mann-Whitney U-test.
ACKNOWLEDGEMENTS
Notes
AUTHOR CONTRIBUTIONS
Conceptualization: all authors.
Data curation: Seung Ki Kim and Eun Sook Park.
Formal analysis: Seung Ki Kim.
Funding acquisition: Eun Sook Park.
Investigation: Han Kyul Park.
Methodology: Han Kyul Park and Eun Sook Park.
Project administration: Eun Sook Park.
Resources: all authors.
Software: Seung Ki Kim.
Supervision: Seung Ki Kim and Eun Sook Park.
Validation: all authors.
Visualization: all authors.
Writing—original draft preparation: all authors.
Writing—review & editing: all authors.
Approval of final manuscript: all authors.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download