CASE
This case involves a 54-year-old lady with no significant past medical history. She presented with symptoms of dyspepsia and gastritis, and was worked up with an ultrasound (US) of the hepatobiliary system. This revealed a 2 cm isoechoic lesion within the pancreatic uncinate process with non-dilated pancreatic and common bile ducts. No gallstones were seen and there were no other abnormalities of the hepato-pancreato-biliary system. (
Fig. 1A) Further investigation with computer tomography (CT) of the pancreas showed a solid hypervascular mass measuring 1.7×1.5×1.3 cm in the posteromedial aspect of the pancreatic uncinate process with no invasion into adjacent structures (
Fig. 1B). Magnetic resonance cholangiopancreatography (MRCP) confirmed the above findings and measured a distance of 4 mm between the uncinate process tumor and the adjacent non-dilated main pancreatic duct (MPD) (
Fig. 1C). Position emission tomography (PET-CT) showed a fluorodeoxyglucose (FDG)-avid mass in the pancreatic uncinate process suspicious for NET with no distant sites of metastasis (
Fig. 1D). An endoscopic retrograde cholangiopancreatography (ERCP) with endoscopic ultrasound (EUS) was performed showing a 1.7×1.0 cm hypoechoeic uncinate process mass with no extension into surrounding structures (
Fig. 2A). Endoscopic findings and histopathological analysis from fine needle aspiration cytology (FNAC) were both highly suspicious for NET. Pre-operative tumor markers (carcinoembryonic antigen, cancer antigen 19-9) were within normal ranges, and serum chromogranin A was 63.5 ng/ml.
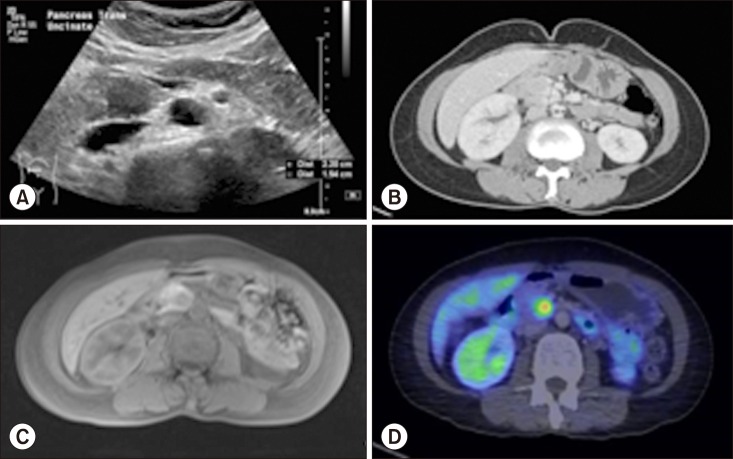 | Fig. 1(A) Ultrasound of the hepatobiliary system showing a 2.2×1.8×1.5 cm isoechoic lesion within the pancreatic uncinate process with non-dilated pancreatic duct. (B) Computer tomography of the pancreas showing a solid hypervascular mass measuring 1.7×1.5×1.3 cm in the posteromedial aspect of the pancreatic uncinate process with no invasion into adjacent structures. There is no suspicious lymphadenopathy identified. (C) Magnetic resonance cholangiopancreatography confirming findings of ultrasound hepatobiliary system and computer tomography of the pancreas with a measured distance of 4 mm between the uncinate process tumor and the adjacent non-dilated main pancreatic duct. (D) Position emission tomography-Computer tomography shoring a fluorodeoxyglucose-avid mass in the pancreatic uncinate process suspicious for neuroendocrine tumor with no distant sites of metastasis.
|
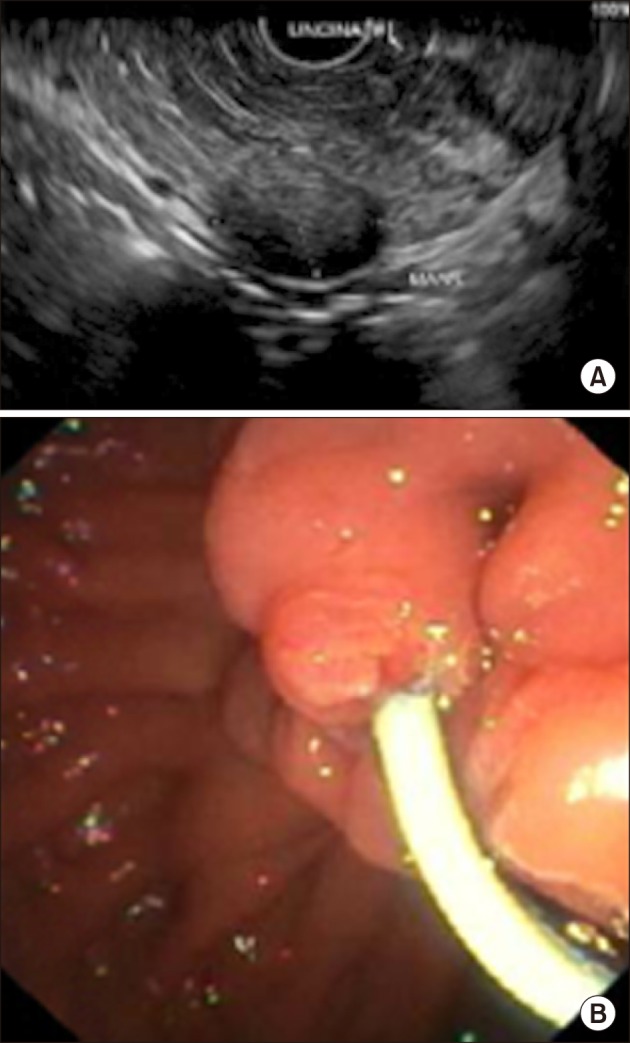 | Fig. 2(A) Endoscopic retrograde cholangiopancreatography with endoscopic ultrasound showing a 1.7×1.0 cm hypoechoeic uncinate process mass with no extension into surrounding structures. (B) 5 French×8 cm Cook Zimmon® single pigtail plastic stent was deployed and unflanged within main pancreatic duct.
|
This patient was counseled for and underwent robotic enucleation of pancreatic uncinate neuroendocrine tumor.
ERCP with endoscopic insertion of an MPD stent was performed prior to surgery. The MPD was cannulated using the sphincterotome with guidewire and deep cannulation was successful with a single pass. Contrast was injected and a pancreatogram obtained. A 5 French×8 cm Cook Zimmon® single pigtail plastic stent was deployed and unflanged (
Fig. 2B). The operative procedure began with insertion of a subumbilical 1.2 cm port using Hasson's technique. 1.2 cm right iliac fossa, 0.8 cm right flank, 0.8 cm left iliac fossa and 0.8 cm left flank ports were then inserted under direct vision. Surgery started with mobilization of the hepatic flexure and opening of the gastrocolic ligament with entrance into the lesser sac. Duodenum was kocherized past the aorta and duodenojejunal flexure mobilized. The gastrocolic trunk was divided and vessel loops placed around the middle colic vessels, superior mesenteric artery and vein. Uncinate process of the pancreas was exposed and lesion marked using intra-operative ultrasound (IOUS), confirmed intra-operatively to be 4 mm away from the MPD and with no extension into gastroduodenal artery, portal vein or bile ducts. Enucleation was completed with Harmonic® scalpel and bipolar electrocautery (
Fig. 3). Large tributaries and supplying vessels were divided between clips and hemostasis achieved with Evicel® and Surgicel Snow®. Omentum was tagged to the cavity and 2 Blake drains were left in-situ. The total operation time was 325 minutes and estimated blood loss was 50 mls.
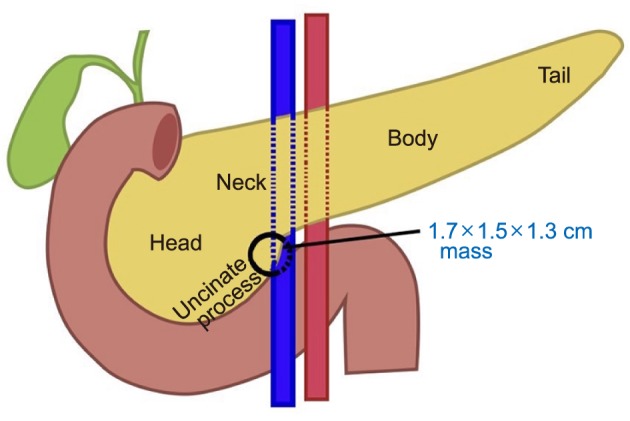 | Fig. 3Relationship of pancreatic uncinate mass to surrounding structure and depicting the extent of resection.
|
This patient's post-operative recovery was complicated by raised serum amylase and high drain output with raised fluid amylase. Somatostatin was started and a CT abdomen pelvis (CTAP) performed on post-operative day (POD) 3 revealed a grossly edematous pancreatic body and tail with peripancreatic fluid, compatible with pancreatitis likely secondary to the pancreatic duct stenting. No other abnormalities were detected in adjacent vessels and solid organs. Our patient was managed conservatively with non-opioidal analgesia and aggressive fluid resuscitation. She had resolution of pancreatitis, normalized serum amylase and downtrending drain output by POD 6. A repeat CTAP was performed on POD 10 for persistently high nasogastric tube output with suspicion of mechanical obstruction and gastroparesis. This revealed a 6.5×6.0×4.1 cm loculated hypodense fluid collection in the surgical bed suggestive of a post-surgical peripancreatic collection. The patient remained hemodynamically stable and was treated with US-guided percutaneous drainage with fluid cultures showing no bacterial growth. She was gradually escalated to feeds on POD 12 and subsequently tolerated diet on POD 14. Nasogastric tube and surgical drains were removed on POD 15 and 16 respectively and a repeat CTAP on POD 24 showed complete resolution of peripancreatic collection. She was discharged stable, afebrile and asymptomatic on POD 18.
Histology revealed a well-differentiated, grade 1 uncinate neuroendocrine tumor, 1.6 cm in size. Mitotic rate was 1/2 mm2 and Ki-67 labeling index was <3%. There was no peripancreatic extension of tumor, tumor necrosis, lymphovascular or perineural invasion. Immunohistochemistry revealed positivity for synaptophysin but negative for chromogranin. On last follow-up 16 months post-operatively, there was no evidence of recurrence and a complete return to functional baseline.
Go to :

DISCUSSION
PNETs are rare neoplasms comprising 5–10% of all diagnosed pancreatic tumors, with an estimated prevalence of 1 in 100,000 people.
1 The diagnosis of this condition has increased six fold over the past decade, largely owing to incidental diagnoses from more frequent use of high resolution radiological imaging for work-up of non-specific abdominal symptoms.
1 Furthermore, the size of diagnosed PNETs has considerably decreased, with a sizeable number being detected at <2 cm (26–61%).
2 PNETs can be divided into NF-PNET and F-PNET tumors (insulinoma, gastrinoma, glucagonoma, VIPoma, somatostatinoma), with the majority belonging to the former group (90%).
1
Mitotic count and Ki-67 expression are widely recognized as the most important factors differentiating between the grades of PNETs, with significant implications on prognosis. While there has been no universally agreed Ki-67 cutoff differentiating the grades of PNETs, the 2017 World Health Organization (WHO) classification states 3% and 20% as being appropriate cutoffs between Grade 1–3 PNETs.
134 Numerous prognostication systems have been proposed for PNETs, with the ones most commonly used today being the WHO 2010 criteria, European Neuroendocrine Tumor (ENET) society guidelines and the American National Comprehensive Cancer Network (NCCN) guidelines. Across most guidelines, the most commonly cited prognostic factors include age, size, lymphatic metastasis, peritumoral invasion, distant metastases, calcification on imaging, absence of symptoms in NF-PNETs, Ki-67 index and mitotic count.
56 There is no gold standard for management of NF-PNETs, with marked differences between a multitude of different guidelines. The European Neuroendocrine Tumor (ENET) Society guidelines states that patients with G1/G2 NF-PNETs <2 cm could be managed conservatively in the presence of pancreatic head localization and no signs of malignancy at initial imaging. These guidelines also mandate biannual EUS and MRCP with re-evaluation for surgery once there is an increase in tumor size of 0.5 cm.
3 In contrast, the American National Comprehensive Cancer Network (NCCN) guidelines suggest conservative management only for incidentally diagnosed, low grade NF-PNETs that are <1 cm.
7 The Canadian National Expert Group advocates a surgical approach for every healthy patient with resectable disease, with surveillance only considered in NF-PNETs smaller than 2 cm with a low Ki-67 index.
8 Arguments for an aggressive approach towards NF-PNETs include statistics suggesting frankly malignant behaviour in a significant (40%) portion of tumors smaller than 2 cm, with thorough histological examination (mitotic and Ki-67 indices) possible only on a resected specimen.
1 This has, however, to be balanced against the considerable morbidity and mortality associated with pancreatic surgery, and long term risks of exocrine and endocrine insufficiency.
The surgical management of PNET is a delicate balance between demolitive (total pancreatectomy, distal pancreatectomy, pancreaticoduodenectomy), and pancreaspreserving surgery (enucleation (EN)). There is no gold standard of guidelines currently that optimizes the morbidity of excessive pancreatic parenchymal removal against benefits of long term disease-free survival (DFS) and overall survival (OS). While there have been sporadic reports of suboptimal results after EN in terms of DFS and OS as compared to demolitive procedures, the vast majority of reviews report no significant differences.
191011 Most recent NCCN guidelines report benign tumors, isolated lesions, distance between tumor and MPD >3 mm with no focal stricture or dilatation, insulinomas and gastrinomas <2 cm in size, and NF-PNETs <2 cm in size with low Ki-67 and mitotic indices as indications for EN procedure.
7 For the group of F-PNETs, EN is advocated only for insulinomas, given the higher risk of lymph node metastasis and locoregional involvement associated with other F-PNET subtypes.
6 Strict adherence to the size criteria for NF-PNETs is strongly advocated in this study, given the undoubted correlation between tumor size and risk of malignancy and metastasis.
7 A common consensus of current data suggests that an EN approach is feasible only for small (<2 cm), low grade, superficial NF-PNETs.
1810 With appropriate selection criteria applied, EN has been shown to have superior perioperative outcomes (operative times, blood loss, adverse events according to Clavien-Dindo grading), and an equivalent DFS and OS when compared to demolitive procedures.
19101112 One major drawback of EN is its consistently significant correlation with higher rates of post-operative pancreatic fistulas (POPF), especially in head of pancreas (HOP) and uncinate PNETs.
1 Postulated explanations include difficulty with suture re-approximation of pancreatic parenchyma after EN, and PNETs being more commonly associated with a non-dilated MPD within a soft and friable pancreas (specifically higher risk in HOP lesions due to the presence of a larger pancreatic duct).
1 Most current guidelines mandate that tumors should be at least 2–3 mm from the MPD to reduce risk of direct injuries and POPF development.
111 This should be evaluated pre-operatively with MRCP, and intra-operatively with IOUS. Fortunately, a large proportion of POPF that do occur are classified as low grade and are most commonly amenable to conservative management.
11213 Enucleation also presents a unique challenge in terms of pancreatic uncinate tumors due to its location and intimate association with important structures (duodenum, pancreaticoduodenal vessels, superior mesenteric vessels) necessitating careful, patient and precise peri-tumoral dissection.
In comparing open and minimally invasive (MIS) approaches to PNET surgery, the latter has been reported to be non-inferior, and significantly associated with shorter operative time, lower morbidity and POPF rates, and decreased duration of hospital stay.
121314 To date there exist only two meta-analyses specific to the comparison between MIS and open approaches for PNETs. Both studies have found MIS to be associated with decreased perioperative complications, less blood loss, shorter length of stay with comparative operative time, POPF incidence and operative mortality rates.
12 In addition, MIS EN was associated with better post-operative outcomes when compared to other MIS parenchymal-preserving procedures (central pancreatectomy, HOP resection, distal pancreatectomy).
111 The vast majority of other well powered studies fail to discriminate non-PNET pathologies, making data poorly translatable to this specific subgroup of patients.
Robotic pancreatic surgery (RPS) has been gaining traction in recent years for the management of both benign and malignant pancreatic conditions. Goh et al.
15 report on the safe adoption of RPS for a variety of hepatopancreatobiliary surgeries (including distal pancreatectomy, subtotal pancreatectomy, pancreato-splenectomy, Puestow procedure, uncinectomy and pancreaticoduodenectomy) with low rates of open conversion (3.3%), POPF (13.3%) and 0 30-day/in-hospital mortalities. While robotic surgery is generally associated with a longer operative time (robot docking/undocking, instrument changing, bias towards a larger proportion of surgeons better versed with the open/laparoscopic approaches as compared to the robotic approach) and increased costs, many argue that this is compensated by lower rates of open conversion, higher rates of splenic preservation, reduced length of hospital stay and quicker return to activities.
1216171819 Most studies now report comparable results between robotic and laparoscopic surgery with regards to both peri-operative, long-term and oncological outcomes.
12171819 It is however widely recognized that there is an overall lack of statistically powered studies comparing the open, laparoscopic and robotic techniques for management of PNETs, especially with regards to long term DFS and OS.
116 Moreover, most current studies have marked heterogeneity with regards to tumor type and surgical procedure (pure robotic vs. pure laparoscopic vs. combination procedures), limiting the feasibility of cogent comparison.
Ore et al.
17 is one of few to have documented a suggested technique for robotic enucleation of PNETs. This technique differed from ours firstly in terms of port placement (5 mm port in the right anterior axillary line to secure the liver retractor, 12 mm port in the right lower quadrant for ultrasound access and needle passage, 12 mm camera port located in proximity to the tumor and three 8 mm robotic ports across the upper abdomen). This report also advocated the use of secretin administration with observation of surgical cavity for signs of pancreatic leak. Apart from this, surgical technique and steps were largely similar between this report and ours.
17 In addition, Ore et al.
17 performed a systematic review revealing shorter operative time, reduced blood loss and no significant differences in morbidity, post-operative stay or POPF rates when robotic EN was compared to open and laparoscopic techniques. Distance between tumor and MPD as well as operative time were reported to be the only significant predictors for POPF in patients undergoing minimally-invasive EN of PNETs.
17
A separate propensity score-matched analysis conducted by Tian et al.
18 found that robotic EN of PNETs for tumors <2 cm did not increase risk for POPF or major post-operative complications as compared to the open approach. In addition, this study found that robotic surgery reduced the duration of surgery and blood loss.
18 Zhang et al.
19 is one of few to have reported on comparisons between robotic and laparoscopic pancreatectomies for PNETs. This study found the robotic approach to be superior with regards to less blood loss, higher rates of splenic preservation and more lymph node harvest. Generalizability is however limited, given that this study only included patients undergoing distal pancreatectomies.
19 Machado et al.
20 reported in November 2018 the first case of robotic resection of pancreatic uncinate process for PNET. The distance between tumor and MPD was 4 mm and this patient had no post-operative complications.
20
To our knowledge, this is the second report of a robotic enucleation of a pancreatic uncinate NET in the current literature. Although the surgical procedure proceeded smoothly with no major intraoperative adverse events and minimal blood loss, the patient's postoperative recovery was complicated by the development of POPF with a symptomatic intra-abdominal collection requiring percutaneous drainage. This in our opinion was not surprising as EN is well-known to be associated with a high rate of POPF of over 50% especially when the tumor is deepseated and located close to the MPD.
111214 Nonetheless, it is important to note that despite the potentially higher postoperative morbidity rate especially with regard to the POPF associated with EN compared to pancreaticoduodenectomy, the incidence of life-threatening POPF such as bleeding from pseudoaneurysm is frequently lower with EN. This has been postulated to be due to the absence of activated pancreatic juice after EN compared to that after pancreaticoduodenectomy whereby the pancreatic fluid has been mixed with and potentially activated by enteric contents. Moreover, pancreaticoduodenectomy when performed for PNET is well-known to be associated with a higher rate of clinically-significant POPF compared to that for ductal adenocarcinoma due to the presence of a small non-dilated pancreatic duct and soft pancreas.
12
Hence, when considering performing EN for uncinate tumors, one should carefully consider the technical challenges of this rare and novel procedure especially via the minimally-invasive approach, combined with the increased incidence of POPF versus the more severe morbidity, mortality and long-term consequences associated with pancreaticoduodenectomy. More comparative studies in larger patient cohorts are needed to determine if EN is a safe and effective alternative parenchymal-saving surgical strategy for small benign and premalignant tumors located in the uncinate process.
Go to :







 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download