Abstract
Background
The most important reason for pre-operative administration of medication is to reduce anxiety. Alleviation of fear and anxiety about surgery enables patients to remain comfortable during treatment. Dexmedetomidine (DEX) is a fast-acting drug that is used as a premedication in different circumstances because it has sedative and anti-anxiolytic effects, and stable hemodynamics. It also has the advantage of intranasal administration. The aim of this study was to investigate the effects and hemodynamic stability of DEX by retrospectively analyzing cases in which DEX was administered nasally as a premedication.
Methods
Ten patients treated at Dankook University Dental Hospital, recruited between February and April 2015, received intranasal delivery of 2 µg/kg DEX, 30 minutes prior to general anesthesia. Anesthesia records of anxiety, blood pressure, respiration, pulse, estimated arterial oxygen saturation (SpO2), and partial pressure, or maximum concentration, of carbon dioxide (ETCO2) were analyzed.
Results
Administration of DEX prior to a general anesthetic effectively relieved anxiety. Respiratory depression, the most severe adverse effect of other sedatives, was not observed. Hemodynamic stability under general anesthesia was maintained during treatment and a reduction in emergence delirium was observed upon completion of treatment.
The most important purpose of pre-operative medication is to alleviate fear and anxiety about surgery, enabling patients to be maintained comfortably during treatment. In particular, pre-operative medication facilitates general anesthesia in children and in patients for whom communication is challenging, or who have had unpleasant or distressing hospital experiences [1]. Ideal pre-operative medications should be fast-acting, rapidly metabolized, and reliable. Patient respiratory function should be maintained and the drug should be neuroprotective, with minimal cardiovascular effects [2]. The desired effects are sedation, anxiety relief, memory suppression, pain relief, decreased secretion in the airway and the stomach, prevention of aspiration pneumonia, vagus nerve blockade, and prevention of nausea, vomiting, and infection.
Dexmedetomidine (DEX) is a strong and fast-acting alpha-2 adrenergic receptor agonist that is used as a premedication in various circumstances due to its sedative and anti-anxiolytic effects and stable hemodynamics. Unlike other sedatives, DEX is compatible with intranasal administration and it rarely induces respiratory depression [1]. However, DEX administration has been reported to be associated with adverse effects, such as bradycardia, hypotension, and awareness at rest [23].
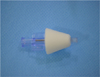
Compared to other sedatives commonly used in pediatric patients, intranasal administration does not require patient cooperation and the intranasal mode of delivery permits the administration of an accurate dose. Rates of absorption and bioavailability are similar to those of drugs administered intravenously, which makes DEX superior to oral sedatives. DEX may be administered easily and painlessly in pediatric patients who do not possess behavioral control [456]. Use of a disposable Mucosal Atomizer Device (LMA MAD Nasal™, Wolfe Tory Medical Inc., USA) to enable aerosol delivery of DEX into the nasal cavity (Fig. 1) is presently being assessed. It is considered that this mode of delivery may reduce the burning sensation and associated coughing that may occur during intranasal administration [5].
The aim of this study was to investigate the effects and hemodynamic stability of DEX by retrospectively analyzing cases of intranasal DEX administration as a premedication prior to general anesthesia for dental treatment.
This study was approved by the Institutional Review Board (IRB) of Dankook University Dental Hospital. Ten pediatric patients who attended the Pediatric Department of Dankook University Dental Hospital between February 2015 and April 2015 and required anxiety relief prior to general anesthesia for dental treatment received intranasal DEX administration. The characteristics of the selected patients are described in Table 1. Written informed consent was obtained from all patients prior to general anesthesia.
Patient charts were reviewed to obtain information about personal details, type of surgery, premedication, and general anesthesia. All patients received intranasal administration of 2 µg/kg DEX, which was delivered by an atomizer 30 minutes before general anesthesia (Fig. 2). After DEX administration, the patient's estimated arterial oxygen saturation (SpO2) was entered into the anesthesia record. The effects of anxiety relief, details of the general anesthetics and muscle relaxants administered, anesthetic time, length of stay in the recovery room, respiratory rate, pulse, blood pressure, SpO2, and partial pressure, or maximum concentration of carbon dioxide (ETCO2) were retrospectively analyzed by reviewing patient anesthesia records.
Patient gender, age, and body weight are presented in Table 1. Surgical methods, anesthetic time and treatment time for each patient are presented in Table 2. Procedures performed under general anesthesia were minor surgeries, such as treatment for dental caries and extraction of mesiodens, which are common procedures in pediatric dentistry. The period of anesthesia ranged from 20 until approximately 180 minutes and treatment time varied between 10 and approximately 140 minutes.
The dose of administered DEX was 2 µg/kg, with a mean dose of 43 µg per patient. Patients began to experience drowsiness about 20 minutes following intranasal administration, with their condition prior to general anesthesia described as sedated and compliant. DEX effectively relieved patient anxiety and there was no incidence of respiratory failure, which is the most severe adverse effect recorded following use of other sedatives.
General anesthesia was induced by constant flow of 3% sevoflurane, which was administered via a warming circuit. Hemodynamically stable blood pressure and heart rate were maintained during treatment (Table 3). Patients remained in the recovery room for an average of 30 minutes post-anesthesia, and less than 1 out of 10 patients showed emergence delirium, indicating the effect of reduced delirium.
Anxiety in pediatric patients is significant problem. Anxiety relief is used to manage a patient's distress before, during and after treatment, and may have a great impact on future treatments [5]. Premedication is given to pediatric patients in order to relieve anxiety prior to administration of a general anesthetic. Midazolam is used widely as a premedication in this context; however, DEX was suggested as an alternative because of adverse effects associated with midazolam use, including emergency delirium, reversibility, and negative behavioral changes after surgery procedure [2].
DEX is a fast-acting alpha-2 adrenergic receptor agonist [123478] with few adverse effects due to its short half-life [8]. Respiratory failure, which is a risk factor of most sedatives, occurs more often in obese patients. Jayaraman et al. [4] reported that there was no incidence of respiratory failure following sedation of obese patients using DEX. Use of sevoflurane in pediatric patients undergoing general anesthesia is associated with a high risk of emergence delirium; although it has been reported that the incidence of emergence delirium is lower when DEX is used [8]. However, this is associated with a temporary increase in blood pressure and reflex bradycardia, with subsequent hypotension. In addition to these adverse effects, nausea, hypoxia, and ventricular tachycardia may also occur [3].
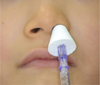
DEX requires intravenous administration, which is not possible for patients who harbor a fear of needles or who are not cooperative. In contrast, intranasal administration enables delivery along the nose-brain pathway, via the olfactory mucosa and the intranasal blood vessels, which provides a fast-acting and useful adjuvant delivery route for sedation [91011]. Instillation and spray are the two methods of intranasal drug administration. Instillation has shortcomings that include nasopharyngeal swallowing and discomfort, whereas intranasal spray can enable more even distribution and absorption across a broader mucosal area than intranasal instillation [9]. The intranasal spray (LMA MAD Nasal™) used in this study was assembled with a disposable syringe and is sprayed in droplets of 20-100 µm, which is broadly distributed over the mucosa (Fig. 3). The advantages of an intranasal spray are the ease of application (in most cases) and ease of use, as it is readily delivered into the nose without any requirement for specific training.
Premedication with intranasal DEX facilitates the sedation process in uncooperative patients, such as patients with autism [12]. Commonly used oral, intramuscular or intravenous sedation are challenging to administer, as they require the cooperation of the patient, which may be difficult if the patient has a fear of needles or minding in medications. These situations occur more often in pediatric patients. In comparison, intranasal administration permits the treating practitioner to control the patient's movement to a certain degree. Delivery of intranasal drugs permits absorption through the nasal mucosa and into the blood stream [9]. This mode of delivery is fast-acting due to the favorable absorption rate and bioavailibility, even for small doses. The burning sensation and coughing that are associated with intranasal administration were recently reported to be diminished if the medication is delivered by a LMA MAD Nasal™ [9]. In this study, pediatric patients undergoing dental treatment under general anesthetic with sevoflurane and desflurane received an intranasal administration of DEX as a premedication. The results showed that blood pressure, respiratory rate, pulse, SpO2, and ETCO2 were all within the normal range for general anesthesia, and hemodynamic stability was observed. No adverse effects, such as bradycardia, hypotension, nausea, hypoxia or ventricular tachycardia occurred following DEX use.
This study demonstrates that patient anxiety is effectively relieved by administration of DEX prior to general anesthesia, and that this does not induce respiratory depression, which is one of the most severe adverse effects of other sedatives. Analysis of patient outcomes after completion of treatment showed that hemodynamic stability was maintained during general anesthesia and emergence delirium was reduced.
Although additional observation and research are required, this study suggests that premedication administration of DEX is safe for use in dental treatment of pediatric patients receiving general anesthesia.
Figures and Tables
Table 1
Details of pediatric patients and procedure dates

Table 2
Details of dental treatments received by pediatric patients requiring general anesthesia
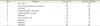
Table 3
Vital signs of pediatric patients
References
1. Linares Segovia B, García Cuevas MA, Ramírez Casillas IL, Guerrero Romero JF, Botello Buenrostro I, Monroy Torres R, Ramírez Gómez XS, et al. Pre-anaesthetic medication with intranasal dexmedetomidine and oral midazolam as an anxiolytic. A clinical trial. An Pediatr (Barc). 2014; 81:226–231.

2. Mahmoud M, Mason K. Dexmedetomidine: review, update, and future considerations of paediatric perioperative and periprocedural applications and limitations. Br J Anaesth. 2015; 115:171–182.

3. Gertler R, Brown HC, Mitchell DH, Silvius EN. Dexmedetomidine: a novel sedative-analgesic agent. Proc (Bayl Univ Med Cent). 2001; 14:13.
4. Jayaraman L, Sinha A, Punhani D. A comparative study to evaluate the effect of intranasal dexmedetomidine versus oral alprazolam as a premedication agent in morbidly obese patients undergoing bariatric surgery. J Anaesthesiol Clin Pharmacol. 2013; 29:179.
5. Korean Academy of Pediatric Dentistry. Pediatric Dentistry. 5th ed. Seoul: Dental Wisdom;2014.
6. Kim J, Kim S, Lee DW, Ryu DS. The alternative of oral sedation for pediatric dental care. J Dent Anesth Pain Med. 2015; 15:1–4.
7. Ambi US, Joshi C, Ganeshnavar A, Adarsh E. Intranasal dexmedetomidine for paediatric sedation for diagnostic magnetic resonance imaging studies. Indian J Anaesth. 2012; 56:587.
8. Liu Y, Kang DL, Na HY, Li BL, Xu YY, Ni J, et al. Consequence of dexmedetomidine on emergence delirium following sevoflurane anesthesia in children with cerebral palsy. Int J Clin Exp Med. 2015; 8:16238.
9. Hussain AA. Mechanism of nasal absorption of drugs. Prog Clin Biol Res. 1988; 292:261–272.
10. Kupietzky A, Houpt MI. Midazolam: A review of its use for conscious sedation in children. Pediatr Dent. 1993; 15:237. 241.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download