Abstract
Vascular injury caused by a central venous catheter (CVC) has been reported to be a rare complication, especially delayed vascular injury due to CVC has a few cases and it can be fatal because of delayed recognition and more serious complications. A 59-year-old woman with no available medical history was admitted for treatment of ovarian cancer. For the surgery, a triple-lumen CVC was placed through the left subclavian vein. Parenteral nutrition through the CVC was used for postoperative nutritional management in the first postoperative day. On the sixth postoperative day (POD), the patient suddenly complained of dyspnea. The CT revealed bilateral pleural effusion and irregular soft tissue density and air bubble in anterior mediastinum suggesting migration of the distal portion of the CVC into the anterior mediastium. In the intensive care unit (ICU) bilateral thoracentesis and percutaneous drainage were performed. She was discharged from the ICU in 3 days later and transferred to the general ward. This case emphasizes the possibility of the delayed vascular injury related to CVC and some strategies for prevention of vascular injury.
The incidence of vascular injury caused by a central venous catheter (CVC) has been reported to be a rare complication with an incidence of 0.17% [1]. But the vascular injury, especially delays in recognition of the injury can be fatal with mortality rates as high as 78% because serious complications can be occurred in process of time. [2,3]. We describe a case of acute mediastinitis after delayed vascular injury by a CVC and total parenteral nutrition (TPN) that occurred 6 days after the initial placement of a CVC.
A 59-year-old woman with no available medical history was admitted to our hospital for treatment of ovarian cancer. She was scheduled to have an operation such as hysterectomy, multiple lymph node dissection and omentectomy. For the surgery, a triple-lumen CVC (BIOLINE NEXT, EWHA Biomedics Co.,Ltd., Korea) was placed through the left subclavian vein because many patients had expressed their discomfort when the CVC was placed through the jugular vein. The CVC was made of latex-free polyurethane and was 7 Fr. in diameter and 15 cm in length. The blood was observed to flow back freely through the all triple-lumen of the catheter. Total volumes of infused fluid in 5 hours of operation time were 3,700 ml of crystalloid and the estimated blood loss was 1,000 ml, urine output was 100 ml/hr. Postoperative chest X-ray showed no specific abnormality and the tip of CVC placed in the superior vena cava (SVC) (Fig. 1). Parenteral nutrition through the CVC was used for postoperative nutritional management in the first postoperative day because of she underwent primary repair of perforated rectum resulting from adhesiolysis between uterus and rectum. A total 1970 ml of commercially available hyperosmolar (1500 mOsm/l) parenteral solution composed of glucose, amino acid, lipid and electrolytes was administered through the CVC per day. On the sixth postoperative day (POD), the patient suddenly complained of dyspnea. No fever was detected and wound of operation site was clear. We ran a computerized tomography (CT) scans to rule out pulmonary thromboembolism. The CT revealed a no evidence of pulmonary thromboembolism and bilateral pleural effusion and irregular soft tissue density and air bubble in anterior mediastinum, suggesting migration of the distal portion of the CVC into the anterior mediastium (Fig. 2). For the close observation, she was transferred to intensive care unit (ICU). In the ICU, bilateral thoracentesis and percutaneous drainage were performed and pleural and mediastinal effusion were drained. A few hours later, she felt that dyspnea was alleviated. In the culture of the pleural and mediastinal effusion, triglyceride (1,468 mg/dl) and inflammatory cells were detected. But there was no detection of the microorganism. As for this result, we confirmed that the effusion was caused by the leakage of the TPN fluid through the migrated CVC. The CVC in left subclavian vein was removed. Ceftriaxon and metronidazole were used to control the infection. She was discharged from the ICU in 3 days later and transferred to the general ward. Bilateral percutaneous drainage were removed after 6 days from the insertion. The patient recovered from her respiratory problems and received the chemotherapy for cancer continually.
This delayed vascular injury has been reported to occur as rare complications after the insertion of CVCs [4]. Mukau et al. [5] retrospectively collected 1058 catheters in 853 patients and reported the incidence of vascular erosions to be 0.4%. In this case, vascular injury and perforation due to CVC occurred at 6 days later, and there are several suggested mechanisms that cause delayed vascular injury. The mechanical irritation from the catheter tip to vessel wall is the first mechanism [6]. The catheter tip abutted on the superior vena cava at an angle of 45 degrees perpendicular to the vessel wall was shown in the postoperative chest X-ray film. Other mechanism is chemical irritation to the intima caused by hyperosmolar solution. These two mechanisms have a major effect on vascular injury and perforation synergistically.
Delayed vascular injury was increased by various risk factors, especially that occur simultaneously [7]. The risk factors reported to date are patient factors such as older age, female gender, patient's underlying conditions or drug therapies such as corticosteroids or administration of cytotoxic agents and iatrogenic factors such as left-sided CVC insertion, large catheter, polyethylene catheter [2,8]. In our case, the risk factors are female gender, left sided CVC insertion and so on. The predominance of previous case reports about vascular erosion occurring in female patients has led to the suggestion that female gender may be a risk factor. This may be owing to the smaller caliber and length of venous structures of women compared with average-sized men increase the probability that a central catheter tip will impinge on a vessel wall or bifurcation [2]. The CVCs inserted through the left subclavian vein or left internal jugular vein are more likely to perforate the SVC, because the left brachiocephalic vein forms a horizontal orientation from the SVC. In Walshe study, the predilection for vascular erosion by left-sided catheters was quantified in terms of relative risk, the risk was 2.9 times higher on the left than on the right [1].
In the previous study, the symptoms related to vascular erosions caused by a CVC appears 2.5-3.6 days after insertion of the CVC [2,9]. The most common signs and symptoms related to vascular erosions and mediastinitis are dyspnea, chest pain, neck swelling, fever, and dilated superficial veins [10]. But these signs and symptoms are not specific, therefore a diagnosis of mediastinis according to vascular erosion can be delayed and misdiagnosis can be made. A delayed diagnosis or misdiagnosis may be a significant factor which contribute to the high mortality and morbidity. There was 12.5% mortality seen in the Duntley study [2], and one out of the five patients died in the Walshe study [1]. In order to compensate these problems, when the suspected signs and symptoms are detected, the radiologic examination must be performed immediately and rule out the other causes related underlying medical and surgical conditions.
For prevention of the delayed vascular injury and mediastinitis, it is very important to establish some strategies. The first strategy is about depth of CVC. The right atrial CVC placement is avoided due to the risk of cardiac tamponade. Though, the proximal CVC placement involves the risk of tip positioning at more acute angle with SVC. According to Peres formulae derived to predict the optimum length of catheter insertion, the lengths of CVC insertion should be height/10 for the right internal jugular vein (RIJ), (height/10) -2 cm for the right subclavian vein (RSC), (height/10) + 4 cm for the left internal jugular vein (LIJ), and (height/10) + 2 cm for the left subclavian vein (LSC) [11]. In another study about optimal insertion depth of CVC in Asian, they recommended depth of CVC insertion should be 15 cm for the RIJ, 14 cm for the RSC, 18 cm for the LIJ and 17 cm for the LSC [12]. Second, checking of return of blood via all lumens of the CVC is important. Third, taking a chest X-ray before and during a TPN should be recommended to detect the delayed vascular injury. Not the least of which is right-sided CVC insertion must be conducted in high risk patients, because left-sided CVC insertion is a definite risk factor and controllable factor.
Figures and Tables
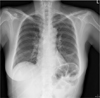 | Fig. 1Chest radiograph showing no specific abnormality and the tip of central venous catheter placed in the superior vena cava. |
 | Fig. 2Computed tomography scan shows bilateral pleural effusion with passive atelectasis of adjacent lung parenchyma and irregular soft tissue density and air bubble in anterior mediastinum suggesting mediastinitis. Red line indicates a displacement of the tip of the central venous catheter out of the superior vena cava. |
References
1. Walshe C, Phelan D, Bourke J, Buggy D. Vascular erosion by central venous catheters used for total parenteral nutrition. Intensive Care Med. 2007; 33:534–537.

2. Duntley P, Siever J, Korwes ML, Harpel K, Heffner JE. Vascular erosion by central venous catheters. Clinical features and outcome. Chest. 1992; 101:1633–1638.
3. Defalque RJ, Campbell C. Cardiac tamponade from central venous catheters. Anesthesiology. 1979; 50:249–252.

4. Jones KW, Seltzer MH, Slocum BA, Cataldi-Betcher EL, Goldberger DJ, Wright FR. Parenteral nutrition complications in a voluntary hospital. JPEN J Parenter Enteral Nutr. 1984; 8:385–390.

5. Mukau L, Talamini MA, Sitzmann JV. Risk factors for central venous catheter-related vascular erosions. JPEN J Parenter Enteral Nutr. 1991; 15:513–516.

6. Valat P, Pellerin C, Cantini O, Jougon J, Delcambre F, Morales P, et al. Infected mediastinitis secondary to perforation of superior vena cava by a central venous catheter. Br J Anaesth. 2002; 88:298–300.

7. Inaba K, Sakurai Y, Furuta S, Sunagawa R, Isogaki J, Komori Y, et al. Delayed vascular injury and severe respiratory distress as a rare complication of a central venous catheter and total parenteral nutrition. Nutrition. 2009; 25:479–481.

8. Bersten AD, Williams R, Phillips G. Central venous catheter stiffness and its relation to vascular perforation. Anaesth Intensive Care. 1988; 16:342–351.

9. Ellis LM, Vogel S, Copeland E. Central venous catheter vascular erosions: diagnosis and clinical course. Ann Surg. 1989; 209:475–478.
10. Marín MR, Rodríguez ME, Buleje JA, Valverde FM, Martínez MM, Pérez PP, et al. Acute mediastinitis due to extravasation of parenteral nutritional formula via a central venous catheter. Am J Crit Care. 2012; 21:296–299.

11. Peres PW. Positioning central venous catheters--a prospective survey. Anaesth Intensive Care. 1990; 18:536–539.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download