Abstract
Prader-Willi syndrome (PWS) is a rare genetic disorder reported rarely in dentistry. Dental practitioners should know the features of PWS because affected patients have a variety of dental symptoms. The current report describes a case of PWS. An 18-year-old male patient presented with traumatic injuries. Initial emergency treatments were performed under sedation, and further treatments were conducted under general anesthesia. After adequate healing, periodic follow-up and dietary management according to the patient's age and nutritional phase were recommended. Dental management of PWS patients consists of active preventive measures in addition to dietary consultation according to age and nutritional phase.
Prader-Willi syndrome (PWS) is a rare neurodevelopmental disease caused by abnormalities on chromosome 15q11.2-q13, and was first described by Prader et al. in 1956 [1]. The prevalence of PWS is 1 in 10,000-30,000 [23]. Among affected individuals, 65-75% of cases are due to a paternal deletion in this region, 20-30% are due to a maternal uniparental disomy 15, and 1-3% are due to an imprinting defect [2]. Conversely, the absence of preferential maternal expression in this region leads to Angelman syndrome [4]. Currently, PWS can be differentiated by DNA methylation analysis, fluorescence in situ hybridization, chromosomal microarrays, and dysmorphic features or clinical characteristics [4].
The most characteristic clinical feature is a gradual change in eating disorders according to age. In early infancy, poor sucking caused by infantile hypotonia leads to anorexia [56]. However, in later infancy or early childhood, hyperphagia caused by suppression of the satiety center leads to obesity [567]. The nutritional phases of PWS are categorized into five main stages and seven subdivisions (Table 1) [6]. Other characteristic features are developmental delay, intellectual disabilities, growth hormone deficiency, growth disorder, sex hormone deficiency, sleep apnea, hypopigmentation, short stature, repetitive and ritualistic behaviors, and skin picking [2567].
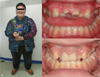
Characteristic orofacial dysmorphic features include narrow bifrontal diameter, almond-shaped palpebral fissures, thin upper lip, downturned corners of the mouth, and drooping shoulders (Fig. 1) [2789]. Greenwood and Small reported extensive periodontal disease in a 12-year-old female PWS patient in 1990 [10]. Salako and Ghafouri reported severe dental caries in a 5-year-old PWS child in 1995 [11]. Recently, Saeves et al. reported that rampant caries can be caused by poor oral hygiene, accompanied by decreased total salivary flow rate and increased salivary viscosity [1213].
Management of PWS consists of hormonal therapy to induce a more normal growth pattern and strict dietary intervention to prevent obesity and diabetes mellitus [256]. In addition, PWS patients need careful follow-up due to intellectual and learning disabilities [256]. Dental management for PWS includes typical dietary interventions along with periodic professional oral healthcare because of the high prevalence of dental caries and periodontal disease caused by difficulties with oral hygiene and changes in salivary composition [121314].
PWS is a rare genetic disease with a characteristic eating disorder and orofacial features, and is rarely reported in dentistry. Recently, we treated a PWS patient under sedation and general anesthesia and report the dental management.
An 18-year-old male patient with PWS presented to our clinic with the chief complaint of crown fracture of the maxillary central incisors caused by a fall. He was diagnosed with PWS, moderate mental retardation, and attention deficit/hyperactivity disorder (ADHD). He had been medicated with Prozac 1 T/day and Concerta 3 T/day, an antidepressant and ADHD medication, respectively. There was no special dental or family history. Clinical and radiographic examinations revealed crown fracture of the maxillary central incisors with pulp involvement and rampant caries (Fig. 1, 2).
He had characteristic features of PWS (Fig. 1). His height was 163 cm, and his weight was 137 kg, with a body mass index (BMI) of 51.6 kg/m2, indicating severe obesity. In the Korean population, the normal BMI range is 18.5-22.9 kg/m2 [15]. With strict dietary intervention and physical exercise, his fasting glucose level was 109 mg/dL and total cholesterol was 225 mg/dL, slightly higher than the normal ranges (76-100 mg/dL and 130-200 mg/dL, respectively). The serum alanine and aspartate aminotransferases and gamma glutamyl transferase were 80 U/L (normal: less than 40 U/L), 59 U/L (normal: less than 40 U/L), and 54 U/L (normal: less than 50 U/L), respectively. Other laboratory values, including blood urea nitrogen (13 mg/dL, normal: 8-23 mg/dL), uric acid (6.3 mg/dL, normal: 4.0-7.0 mg/dL), and triglycerides (78 mg/dL, normal: 50-150 mg/dL), were within normal limits. A chest radiograph, electrocardiogram, and other laboratory values were unremarkable.
At the first treatment, characteristic behavior problems were observed, such as temper tantrums, impulsivity, stubbornness, and aggression. Thus, emergency treatment of the traumatic injuries and further treatments under general anesthesia were planned. Midazolam 30 mg was administered by intramuscular injection to obtain adequate sedation. Then, pulp extirpation of the right maxillary central incisor was performed. Because of poor cooperation, only a temporary glass ionomer filling of the left maxillary central incisor was performed.
A child and adolescent psychiatric clinic was consulted regarding general anesthesia. A fistula formed on the apical area of the maxillary left central incisor while awaiting general anesthesia. Due to poor cooperation, intramuscular ketamine hydrochloride 400.0 mg was administered to induce anesthesia. The patient's electrocardiogram, oximetry, end-tidal CO2, and temperature were monitored. Intravenous cisatracurium 16.0 mg was administered with remifentanil hydrochloride 1 mg in normal saline 100 ml. The patient was intubated nasally. Anesthesia was maintained with sevoflurane, oxygen, and air. After an adequate level of general anesthesia was obtained, root canal and prosthodontic treatment of the right and left maxillary central incisors were performed. Multiple restoration treatments and extractions of the left maxillary third molar and left mandibular third molar were also performed. The treatment time totaled 140 minutes and the anesthesia time was 200 minutes. There was no blood loss or urine output. The patient was transferred to the recovery room after endotracheal extubation when he recovered spontaneous breathing. A few days later, under conscious sedation with midazolam 15 mg by intramuscular injection, prosthodontic crowns were placed (Fig. 1).
After all of the planned treatments were completed, a periodic 3-month follow-up examination was recommended. Continuous dietary intervention and physical activities were also strongly recommended.
PWS is a rare genetic disorder with characteristic gradual eating disorder changes according to age. Dentists should know about the features of PWS because affected patients have various dental symptoms. Traumatic injuries in PWS patients are common due to hypotonia. Various congenital tooth malformations are also common. Rampant caries occurs readily due to poor oral hygiene arising from intellectual disabilities accompanied by frequent food intake, decreased salivary flow, and increased salivary viscosity. Additionally, conscious sedation or general anesthesia is typically needed because of poor cooperation.
Almost all PWS children will become obese if they follow the recommended daily allowance (RDA) for calories or if given a "typical" toddler diet. Thus, patients need to be restricted to 60-80% of RDA to prevent obesity. This patient was identified as being in nutritional phase 3. His height and weight were 163 cm and 137 kg, and his BMI was 51.6 kg/m2, indicating severe obesity. His age corresponded to the 8-years-to-adulthood range of phase 3 (Table 1) [6]. According to the parents, he had a history of temper tantrums over food. In this stage, patients rarely feel full, continuously seek food, and have temper tantrums related to food. These patients are restricted to 50-70% of the RDA to maintain weight. Dentists should advise appropriate dietary measures according to the patient's nutritional phase and consultation with physicians [5].
The patient presented due to a traumatic injury and was also found to have rampant caries. Frequent traumatic injuries in PWS patients result from hypotonia.
Due to poor cooperation, sedation and general anesthesia were carried out. At the first treatment, the depth of anxiolytic sedation was minimal, even at a dosage of more than 0.2 mg/kg of midazolam. At the second treatment, conscious sedation was performed with a lower dosage than that administered for previous emergency treatment. At the first treatment, pain and an unfamiliar environment may have influenced the depth of sedation.
PWS patients need careful assessment because of several risk factors for general anesthesia. First, infantile hypotonia increases the possibility of delayed restoration of spontaneous respiration and pulmonary complications. Thus, muscle relaxants should be chosen and used with care. Moreover, obesity, micrognathia, a high palatal vault, and scoliosis may make airway management difficult. Complications such as acute respiratory failure and pulmonary aspiration can arise unless proper airway management is achieved. Also, because of hyperphagia, preoperative fasting may not be achievable, especially with intellectual disabilities and temper tantrums. Thus, caregivers should monitor their patients. Beyond that, there are difficulties in blood glucose maintenance because of changes in carbohydrate and lipid metabolism. Moreover, many children affected by neurodevelopmental disorders also have thermoregulation disturbances. As with such cases, patients may also have underlying diseases such as cardiac disorders and ADHD, and may be receiving drugs for these conditions. Thus, close consultation with specialists and anesthesiologists is essential.
In conclusion, dental treatment was properly performed under sedation and general anesthesia. Dental management of PWS patients consists of active preventive treatment, along with dietary consultation according to the patient's nutritional phase. Behavior management with sedatives can be considered, and consultations with the patient's physicians are essential given other systemic conditions in these patients.
Figures and Tables
Fig. 1
(A) Characteristic facial features including narrow forehead, thin upper vermillion, and downturned corners of the mouth were observed. The patient also had a typical body habitus, with narrow shoulders and obesity, (B) Preoperative clinical examination revealed crown fracture of the maxillary central incisors with pulp involvement, (C) The postoperative photograph shows prosthodontic treatment of the right and left maxillary central incisors.
References
1. Prader A, Labhart A, Willi H. Ein syndrome von adipositas, kleinwuchs, kryptorchismus und oligophrenie nach myatonieartigem zustand im neugeborenenalter. Schweiz Med Wschr. 1956; 86:1260–1261.
2. Cassidy SB, Schwartz S, Miller JL, Driscoll DJ. Prader-willi syndrome. Genet Med. 2012; 14:10–26.

3. Powis L, Oliver C. The prevalence of aggression in genetic syndromes: A review. Res Dev Disabil. 2014; 35:1051–1071.

4. Buiting K. Prader-willi syndrome and angelman syndrome. Am J Med Genet C Semin Med Genet. 2010; 154C:365–376.

5. McAllister CJ, Whittington JE, Holland AJ. Development of the eating behaviour in prader-willi syndrome: Advances in our understanding. Int J Obes (Lond). 2011; 35:188–197.

6. Miller JL, Lynn CH, Driscoll DC, Goldstone AP, Gold JA, Kimonis V, et al. Nutritional phases in prader-willi syndrome. Am J Med Genet A. 2011; 155A:1040–1049.

7. Whittington J, Holland A. Neurobehavioral phenotype in prader-willi syndrome. Am J Med Genet C Semin Med Genet. 2010; 154C:438–447.

8. Reus L, Zwarts M, van Vlimmeren LA, Willemsen MA, Otten BJ, Nijhuis-van der Sanden MW. Motor problems in prader-willi syndrome: A systematic review on body composition and neuromuscular functioning. Neurosci Biobehav Rev. 2011; 35:956–969.

9. Bailleul-Forestier I, Verhaeghe V, Fryns JP, Vinckier F, Declerck D, Vogels A. The oro-dental phenotype in prader-willi syndrome: A survey of 15 patients. Int J Paediatr Dent. 2008; 18:40–47.

10. Greenwood RE, Small IC. Case report of the prader-willi syndrome. J Clin Periodontol. 1990; 17:61–63.

11. Salako NO, Ghafouri HM. Oral findings in a child with prader-labhart-willi syndrome. Quintessence Int. 1995; 26:339–341.
12. Saeves R, Nordgarden H, Storhaug K, Sandvik L, Espelid I. Salivary flow rate and oral findings in prader-willi syndrome: A case-control study. Int J Paediatr Dent. 2012; 22:27–36.

13. Saeves R, Espelid I, Storhaug K, Sandvik L, Nordgarden H. Severe tooth wear in prader-willi syndrome. A case-control study. BMC Oral Health. 2012; 12:12.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download