Abstract
Anxiety and phobia in dental procedures are common deterrents for patients visiting the dental care unit. For these individuals, procedural sedation may aid in completion of dental treatments. In most cases, the patients are conscious during sedation, thereby allowing spontaneous ventilation. Intravenous sedation (IVS) is widely used during dental treatment to relieve patient anxiety. IVS is the most effective route of administration to achieve this goal, but it requires advanced training, more than that provided during undergraduate education. During IVS, rapid onset, repetitive drug administration, easy titration, and rapid recovery from sedation can be achieved. However, conscious sedation during IVS can result in deep sedation that can cause respiratory and cardiovascular depression. Therefore, the characteristics of intravenous sedatives should be known. The purpose of this review is to discuss the characteristics and usage of intravenous sedatives currently used for dental procedures.
Many patients suffer from anxiety and fear during dental treatment, which are the main reasons why patients avoid visiting the dental care unit [1]. Intravenous sedation (IVS) has allowed many of these patients to receive the necessary dental treatment. IVS is used for patients with circulatory complications for stabilization of their hemodynamic condition during treatment [2].
During IVS, the general goal of sedation is conscious sedation, which is a drug-induced depression of consciousness during which patients respond purposefully to verbal commands, either alone or accompanied by light tactile stimulation. In addition, interventions are required to maintain a patent airway, and spontaneous ventilation is adequate. All providers of conscious sedation services are responsible for ensuring that the environment in which care is delivered is appropriate for the needs and safety of patients [3].
Application of IVS has many advantages as well as some risks, such as respiratory depression, pulmonary aspiration, and cardiovascular depression during dental treatment under conscious sedation [4]. Therefore, clinical and instrumental monitoring, relevant for the patient's medical status and the clinical setting, must be adopted, and it is essential that the individual performing the sedation can recognize the adverse events and manage them appropriately and in a safe manner [5]. For proper IVS, the characteristics of intravenous sedatives should be known. The purpose of the present review was to study the characteristics and usage of intravenous sedatives currently used for dental procedures.
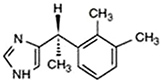
Midazolam is a derivative of benzodiazepine and is widely used in dental sedation. It acts by activating the γ-aminobutyric acid (GABA)A receptor complex and enhancing GABA-mediated chloride currents, thereby leading to hyperpolarization of neurons and decrease in the excitability [6]. It decreases anxiety with minimum respiratory depression and cardiovascular instability [7]. One characteristic of midazolam is that it has desirable anterograde amnesic properties [8].
The onset of action is rapid with midazolam: usually, the peak effect is reached within 2-3 min of administration. After IV injection, midazolam rapidly spreads, with a distribution half-life of 6-15 min because of rapid tissue uptake. Return to baseline values in objective neurological tests was reported 1.5 h after IV administration. The elimination half-life ranged from 1.7 to 3.5 h. The pharmacological duration of action is normally 60-120 min [910].
Paradoxical excitements (or a disinhibitory reaction) occur after IV midazolam administration. The reactions that occur include hallucinations, disorientation, uncontrollable crying or verbalization, agitation, restlessness, involuntary movement, self-injury, and aggressive or violent behavior. These reactions normally occur within 5 min of IV injection and are not likely to resolve spontaneously over time. In this situation, rapid tranquillization is necessary [11]. Administration of additional midazolam is not effective, but rather results in delayed awakening. Low dose ketamine (0.5 mg/kg), propofol, physostigmine, haloperidol, and flumazenil (0.3-0.5 mg) have been reported to be effective [12].
Midazolam is the only benzodiazepine approved by the United States Food and Drug Administration for use in neonates, in whom the half-life is significantly prolonged (6-12 h). Severe hypotension is known to occur in neonates after bolus administration.
For sedation, midazolam 0.5-1 mg (0.01–0.1 mg/kg for pediatric patients) over 2 min can be administered repeatedly, and the sedative effect should be evaluated 2-5 min after each dose adjustment. No single dose should exceed 2.5 mg. A total dose > 5 mg is usually not required to achieve the desired sedation [13].
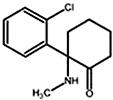
Propofol is one of the most commonly used sedative agents for IVS during dental procedures. Propofol acts by facilitating inhibitory neurotransmission mediated by GABAA receptor binding, and allosterically increasing the binding affinity of GABA for the GABAA receptor [14]. It contains soybean oil, glycerol, and egg lecithin, which causes pain during injection. The pain can be reduced by prior injection of lidocaine (0.5 mg/kg) or mixing lidocaine with propofol (2 mL of 1% lidocaine in 18 mL propofol) or using larger catheters placed in larger veins [15]. Propofol formulation can support the growth of bacteria and should be administered within 6 h of opening the ampule.
The time to peak effect of propofol is 90-100 s after intravenous administration. The distribution half-life is 2-8 min and the elimination half-life is 4-7 h. The context-sensitive half-time for infusions of up to 8 h is less than 40 min [16]. Therefore, recovery from propofol is rapid even after prolonged infusion, which makes it a good agent for outpatient anesthesia. A smaller dose is recommended in elderly patients because of the limited distribution volume.
One of the most common adverse effects observed during propofol sedation is a fall in blood pressure [17]. Propofol inhibits the hypoxic ventilation drive and usually causes airway obstruction and apnea [18]. Propofol is known to be the best IV drug for pediatric sedation, but its use in infants and children is still controversial. Due to its narrow therapeutic range and the vulnerability of children to the sedative effects, administration of propofol may quickly lead to unintended deep sedation even after small dosage increases. Therefore, only educated and qualified personnel should administer propofol for sedation.
Propofol is administered by slow injection, at an initial loading dose of 0.5-1 mg/kg IV, followed by doses of 0.5 mg/kg IV every 3-5 min, as required, until the appropriate level of sedation is achieved [19]. One reasonable approach for administration is to administer 20 mg every 10 s. The infusion rates required for sedation are 25-75 µg/kg/min [20]. The safety of propofol usage is not established in pregnant women and pediatric patients less than 3 years of age.
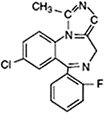
Ketamine, a derivative of the psychedelic drug phencyclidine produces dissociative anesthesia or dissociative sedation depending on the dosage and route of administration. Sedation is characterized by a state of profound analgesia, amnesia, catalepsy, retention of protective airway reflexes, spontaneous respirations, and cardiopulmonary stability [21].
The onset of action and peak plasma concentration of ketamine occur about a minute after intravenous administration. The distribution half-life is 10-15 min and the elimination half-life is 2-3 h. Ketamine is highly lipid-soluble, and only 12% of the agent is bound to proteins so that it rapidly crosses the blood-brain barrier. The duration of sedation is dose-dependent and is usually 5-10 min when administered intravenously. Therefore, ketamine is more suitable for sedation during short procedures [22].
Compared to other sedative agents, which may have a depressive effect, mild cardiovascular stimulation occurs when ketamine is administered. Ketamine increases the heart rate, cardiac output, and blood pressure, and it should not be used in patients with hypertension and at a risk of cerebrovascular accidents and ischemic heart disease. It is the only sedative agent that can maintain the functional residual capacity of the lungs during sedation, and therefore, decreases the chances of intra-operative hypoxemia. Ketamine can be used for asthmatic patients, because it causes bronchodilation and does not induce histamine release [23]. However, ketamine can provoke salivary and trachea-bronchial secretions, and laryngospasms have been reported when ketamine was used at high doses in children younger than 3 months of age. In addition, ketamine has a recognized analgesic effect, which is important for dental sedation [24].
The typical adverse effect of ketamine is the emergence phenomenon that was observed in less than 5% of patients in some studies and in approximately 1% of children [25]. The phenomenon may include vivid dreams, floating sensation, hallucinations, and delirium. The incidence is related to the dosage as well as the rate of administration of the drug.
For sedation, reported IV doses of ketamine range from 0.25-0.5 mg/kg; however, majority of the evidence for sedation involves the use of ketamine in combination with other drugs [26].
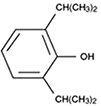
Dexmedetomidine is a potent, highly selective α-2 adrenoceptor agonist with high selectivity for α-2 compared to the α-1 receptor. It causes anxiolysis and sedation via receptors within the locus ceruleus, analgesia via receptors in the spinal cord, and attenuation of the stress response with no significant respiratory depression. Activation of these receptors in the central nervous system leads to inhibition of sympathetic activity, which causes decrease in the blood pressure and heart rate, decreased arousal, sedation, and anxiolysis [27]. It induces a unique sleep pattern that is similar to normal physiological sleep, resulting in easy arousal even by verbal stimuli [28].
The pharmacokinetics of dexmedetomidine are not influenced by renal impairment (creatinine clearance < 30 ml/min) or age. The elimination half-life of dexmedetomidine is 2-3 h, with a context-sensitive half-life ranging from 4 min after a 10-min infusion to 250 min after an 8-h infusion [29].
Dexmedetomidine infusion results in moderate decreases in the heart rate and systemic vascular resistance, consequently resulting in hypotension, bradycardia, and heart blockage, or asystole in severe cases. A bolus injection may produce transient increases in systemic blood pressure and pronounced decreases in heart rate. The effects of dexmedetomidine on the respiratory system include a small to moderate decrease in tidal volume and very little change in the respiratory rate [30].
For sedation, loading doses of 0.5-1 µg/kg for 10 min have been used. Omission of the bolus dose or infusion of a lower dose has caused fewer episodes of severe bradycardia and other hemodynamic perturbations. Infusion rates of 0.1-1 µg/kg/h are generally needed to maintain adequate sedation [31]. In Korea, the use of dexmedetomidine is not approved in pediatric patients.
IVS has many advantages such as rapid onset, repetitive administration, easy titration, and rapid recovery (Table 1). However, some adverse events may occur, including deep sedation, hypoxia, cardiovascular depression, and venous irritation. Therefore, for safe sedation, knowledge regarding the time (including the onset time), peak effect, and duration of action of each drug is essential (Table 2). A dentist using conscious sedation should be able to manage deep sedation and its associated risks. Further, the dentist must attend to the patient until he meets the criteria for discharge and is discharged from the facility.
Generally, single drugs are easier to titrate to achieve the desired effect, and safer than sequential administration of two or more drugs. Drugs used in combination may produce synergistic effects, have different onset times, peak effects, and may be unpredictable or difficult to titrate. Therefore, continuative administration of various sedative drugs should be avoided in IVS for conscious sedation.
Figures and Tables
Table 1
Chemical structures and pharmacokinetic characteristics of sedation drugs

Table 2
Clinical uses of sedation drugs

Acknowledgments
This study was supported by 2016 Clinical Research Grant, Pusan National University Dental Hospital.
References
1. Coolidge T, Irwin SP, Leyster KA, Milgrom P. Determinants of receiving intravenous sedation in a sample of dentally-fearful patients in the USA. SAAD Dig. 2012; 28:52–60.
2. Sago T, Harano N, Chogyoji Y, Nunomaki M, Shiiba S, Watanabe S. A nasal high-flow system prevents hypoxia in dental patients under intravenous sedation. J Oral Maxillofac Surg. 2015; 73:1058–1064.

3. Standing Dental Advisory Committee. Conscious sedation in the provision of dental care: New guidelines. SAAD Dig. 2004; 21:20–22.

4. Ikeda H, Ayuse T, Oi K. The effects of head and body positioning on upper airway collapsibility in normal subjects who received midazolam sedation. J Clin Anesth. 2006; 18:185–193.
5. Urman RD, Punwani N, Shapiro FE. Patient safety and office-based anesthesia. Curr Opin Anaesthesiol. 2012; 25:648–653.
6. Winsky-Sommerer R. Role of GABAA receptors in the physiology and pharmacology of sleep. Eur J Neurosci. 2009; 29:1779–1794.

7. Zacharias M, Hunter KM, Luyk NH. Patient-controlled sedation using midazolam. Br J Oral Maxillofac Surg. 1994; 32:168–173.

8. Coulthard P, Sano K, Thomson PJ, Macfarlane TV. The effects of midazolam and flumazenil on psychomotor function and alertness in human volunteers. Br Dent J. 2000; 188:325–328.

10. Greenblatt DJ, Divoll M, Abernethy DR, Locniskar A, Shader RI. Pharmacokinetics of benzodiazepine hypnotics. Pharmacology. 1983; 27:Suppl 2. 70–75.

11. Golparvar M, Saghaei M, Sajedi P, Razavi SS. Paradoxical reaction following intravenous midazolam premedication in pediatric patients - a randomized placebo controlled trial of ketamine for rapid tranquilization. Paediatr Anaesth. 2004; 14:924–930.

12. McKenzie WS, Rosenberg M. Paradoxical reaction following administration of a benzodiazepine. J Oral Maxillofac Surg. 2010; 68:3034–3036.

13. Martin G, Glass PS, Breslin DS, MacLeod DB, Sanderson IC, Lubarsky DA, et al. A study of anesthetic drug utilization in different age groups. J Clin Anesth. 2003; 15:194–200.

14. Krasowski MD, Nishikawa K, Nikolaeva N, Lin A, Harrison NL. Methionine 286 in transmembrane domain 3 of the GABAA receptor beta subunit controls a binding cavity for propofol and other alkylphenol general anesthetics. Neuropharmacology. 2001; 41:952–964.

15. Swanson ER, Seaberg DC, Mathias S. The use of propofol for sedation in the emergency department. Acad Emerg Med. 1996; 3:234–238.

16. Hughes MA, Glass PS, Jacobs JR. Context-sensitive half-time in multicompartment pharmacokinetic models for intravenous anesthetic drugs. Anesthesiology. 1992; 76:334–341.
17. Jalota L, Kalira V, George E, Shi YY, Hornuss C, Radke O, et al. Prevention of pain on injection of propofol: Systematic review and meta-analysis. BMJ. 2011; 342:d1110.
18. McNeir DA, Mainous EG, Trieger N. Propofol as an intravenous agent in general anesthesia and conscious sedation. Anesth Prog. 1988; 35:147–151.

19. Miner JR, Burton JH. Clinical practice advisory: Emergency department procedural sedation with propofol. Ann Emerg Med. 2007; 50:182–187. 187.e1

20. Jacobi J, Fraser GL, Coursin DB, Riker RR, Fontaine D, Wittbrodt ET, et al. Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill adult. Crit Care Med. 2002; 30:119–141.
21. Green SM, Roback MG, Kennedy RM, Krauss B. Clinical practice guideline for emergency department ketamine dissociative sedation: 2011 update. Ann Emerg Med. 2011; 57:449–461.
22. Wood MN, Manley MC, Bezzina N, Hassan R. An audit of the use of intravenous ketamine for paediatric dental conscious sedation. Br Dent J. 2015; 218:573–577.

23. Krauss B, Green SM. Sedation and analgesia for procedures in children. N Engl J Med. 2000; 342:938–945.

24. Haas DA, Harper DG. Ketamine: A review of its pharmacologic properties and use in ambulatory anesthesia. Anesth Prog. 1992; 39:61–68.

25. Green SM, Johnson NE. Ketamine sedation for pediatric procedures: Part 2, review and implications. Ann Emerg Med. 1990; 19:1033–1046.

26. Wood M. The use of intravenous midazolam and ketamine in paediatric dental sedation. SAAD Dig. 2013; 29:18–30.

28. Venn RM, Bradshaw CJ, Spencer R, Brealey D, Caudwell E, Naughton C, et al. Preliminary UK experience of dexmedetomidine, a novel agent for postoperative sedation in the intensive care unit. Anaesthesia. 1999; 54:1136–1142.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download