Abstract
Orofacial pain is a common complaint of patients that causes distress and compromises the quality of life. It has many etiologies including trauma, interventional procedures, nerve injury, varicella-zoster (shingles), tumor, and vascular and idiopathic factors. It has been demonstrated that the sympathetic nervous system is usually involved in various orofacial pain disorders such as postherpetic neuralgia, complex regional pain syndromes, and atypical facial pain. The stellate sympathetic ganglion innervates the head, neck, and upper extremity. In this review article, the effect of stellate ganglion block and its mechanism of action in orofacial pain disorders are discussed.
Orofacial pain is a common complaint of patients that causes distress and compromises the quality of life. It has many etiologies including trauma, interventional procedures, nerve injury, varicella-zoster (shingles), tumor, and vascular and idiopathic factors. Orofacial pain is often complicated by psychological factors and may require multidisciplinary therapy. The medical history and physical examination, laboratory testing, and imaging findings enable selection of the optimal choice of therapy [12]. It has been shown that the sympathetic nervous system is usually involved in various pain disorders such as postherpetic neuralgia, complex regional pain syndrome (CRPS), and orofacial pain [345]. The stellate sympathetic ganglion innervates the head, neck, and upper extremity. Stellate ganglion block (SGB) can dilate blood vessels in the head, neck, and upper extremities, resulting in an increased blood supply [67]. Preclinical and clinical studies recently revealed that SGB induces sedative effects [89]. In this review article, the effect of SGB and its mechanism of action in orofacial pain disorders are discussed.
Understanding of the anatomy of the stellate ganglion is important for increased therapeutic efficacy and decreased side effects in SGB. The stellate ganglion is located on the transverse process of the C7 vertebra, just below the subclavian artery. It is composed of inferior cervical sympathetic ganglion and the first thoracic sympathetic ganglion. Therefore, the sympathetic nerves that innervate the head, neck, and upper extremity pass through the stellate ganglion [10]. Traditionally, SGB has been performed blindly by palpating the anterior tubercle of the transverse process of C6 (Chassaignac tubercle) to avoid intravascular penetration and then injecting 7-10 ml of local anesthetic. The injectate spreads along the longus colli to the stellate ganglion [11]. Ultrasound-guided SGB improves the safety of the procedure by direct visualization of vascular structures and the target structure (Fig. 1). Additionally, ultrasound-guided SGB improves the quality of the block and requires less volume of local anesthetic than traditional blind technique [12].
The exact role of the sympathetic nervous system (SNS) in the development of neuropathic pain remains unclear. However, many studies have shown that the SNS is closely associated with neuraxial pain. Brief activation of the SNS normally suppresses acute pain by descending inhibition of pain transmission in healthy subjects [13]. In addition, the SNS might be involved in peripheral inflammation and nociceptive activation [1415]. The dendritic cells, which have an important role in innate immunity, respond to norepinephrine (NE) released by the SNS, leading to downregulation of the release of inflammatory cytokines [14]. However, if recruited or adjacent immune cells express α1-adrenergic receptors, activation of the SNS would presumably cause pain and other inflammatory pheromone-based reactions by increasing the release of pro-inflammatory cytokines, resulting in amplification of immune reactions [15]. NE released by the SNS can sensitize peripheral nociceptors (Fig. 2). Following nerve injury, sympathetic axons sprout into the site of the injured nerve. Injured and nearby uninjured axons begin to express α 2-adrenoreceptors, which make these axons sensitive to catecholamines [16]. The pain that is relieved by sympathetic block is classified as sympathetically-mediated pain (SMP). The plasma concentrations of NE and its metabolites are lower in patients with SMP. These findings suggest an increased sensitivity to catecholamines in patients with SMP [17].
As abnormal contact develops between the SNS and the sensory system following peripheral nerve injury, sympathetic nerve block may decrease pain by directly inhibiting afferent nociceptive stimuli traveling via sympathetic pathways [16]. The stellate ganglion has extensive neuronal connections to the hypothalamus and amygdala, and to the infralimbic, insular, and ventromedial temporal cortical regions [17]. A fundamental action mechanism of SGB is to block the sympathetic nerves of the head, neck, and upper extremity, which can improve blood supply to these parts [67]. In addition, SGB is effective for treating SMP in the head, neck, and upper extremity [345]. The analgesic mechanism of SGB can be explained as blocking of the neuronal connections in its sphere of innervation [1920]. Recent preclinical and clinical studies found that SGB has sedative effects [89]. The sedative effects of SGB can be therapeutic in various pain disorders. It was found that SGB decreased concentrations of adrenal hormones in the plasma and/or brain [18] and allowed the normal melatonin rhythm to be reestablished, which may reduce pain intensity [20].
Many studies have shown that the SNS is involved in the development of various orofacial pain disorders such as post herpetic neuralgia, postoperative pain, atypical facial pain, and orofacial neuralgia [1921222324]. Orofacial pain disorders can be very distressing and may be resistant to conservative treatment, leading to depression and anxiety in affected patients [25]. Although the analgesic mechanism of SGB is not clear, many clinical case studies have suggested that it can be an effective option for diagnosis and/or treatment of orofacial pain disorders. Recently, SGB was reported to be effective for treatment of anxiety disorder and posttraumatic stress disorder [18]. In a clinical study, SGB significantly decreased electroencephalogram activity leading to sedation [9]. In an animal study, SGB significantly reduced substance P in the spinal cord, leading to a decrease in nociceptive responses to injection of formalin [26]. Therefore, this sedative and antidepressant effect of SGB might relieve symptoms of orofacial pain disorders. It was reported that a series of SGBs were effective in treating CRPS caused by trauma to the craniofacial region [3]. It was observed that SGB therapy may be useful for the treatment of prolonged postoperative or post procedure pain [52122]. Kojitani et al. reported that SGB added to amitriptyline medication successfully alleviated neuropathic pain after simple tooth extraction [5]. Matsuura et al. performed SGB 2 times a week in 35 patients with postoperative ocular pain that was resistant to anti-inflammatory drugs [22]. It was found that SGB, performed an average of 5.9 times, was effective for 96.6% of patients with nociceptive pain [22]. In a report by Lynch et al., SGB was performed in 14 patients with orofacial neuropathic pain. They observed that five patients noted 50-100% improvement in pain severity 12 months after SGB [19]. Chronic stimulation of the nociceptive system may lead to central sensitization, demyelination, and axonal dysfunction [27]. This can subsequently result in atypical facial pain. It is known that SGB is effective in treating intractable atypical facial pain [242528] and/or burning mouth syndrome [29]. Recently, it was suggested that a trial of SGB in the early stages of various orofacial pain disorders could result in greater reduction in pain severity [213031]. It was also suggested that SGB could prevent facial nerve damage caused by herpes zoster and postherpetic neuralgia that did not respond to medication including acyclovir, steroids, and antidepressants [31].
Figures and Tables
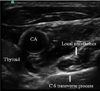 | Fig. 1Stellate ganglion block using ultrasound-guided technique. Local anesthetic was injected at the C6 transverse process. CA: carotid artery. |
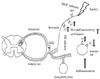 | Fig. 2The sympathetic nervous system (SNS) and pain. Inflammation activates immune dendritic cells. β-2 receptors are downregulated and α-1 receptors are up-regulated on these immune cells. Following nerve injury, functional adrenoreceptors are expressed on peripheral nociceptors. Activation of the SNS increases the level of norepinephrine (NE), which activates α-adrenoreceptors on the afferent fibers, and releases nerve growth factor (NGF). NGF sensitizes peripheral nociceptors through trk A receptors. |
References
1. Sardella A, Demarosi F, Barbieri C, Lodi G. An up-to-date view on persistent idiopathic facial pain. Minerva Stomatol. 2009; 58:289–299.

2. Sarlani E, Balciunas BA, Grace EG. Orofacial pain--Part I: Assessment and management of musculoskeletal and neuropathic causes. AACN Clin Issues. 2005; 16:333–346.

3. Melis M, Zawawi K, Badawi E, Lobolobo S, Metha N. Complex regional pain syndrome in the head and neck: A review of the literature. J Orofac Pain. 2002; 16:93–104.

4. Wu CL, Marsh A, Dworkin RH. The role of sympathetic nerve blocks in herpes zoster and postherpetic neuralgia. Pain. 2000; 87:121–129.

5. Kohjitani A, Miyawaki T, Kasuya K, Shimada M. Sympathetic activity-mediated neuropathic facial pain following simple tooth extraction: A case report. Cranio. 2002; 20:135–138.

6. Kang CK, Oh ST, Chung RK, Lee H, Park CA, Cho ZH, et al. Effect of stellate ganglion block on the cerebrovascular system. Anesthesiology. 2010; 113:936–944.
7. Treggiari MM, Romand JA, Martin JB, Reverdin A, Rufenacht DA, de Tribolet N. Cervical sympathetic block to reverse delayed ischemic neurological deficits after aneurysmal subarachnoid hemorrhage. Stroke. 2003; 34:961–967.
8. Jeong S, Jeon Y, Yeo J, Baek W. The effects of stellate ganglion block on the electroencephalogram in rats. J Anesth. 2014; 28:601–605.

9. Yeo J, Jeon Y. Effects of stellate ganglion block on sedation as assessed by bispectral index in normal healthy volunteers. Pain Physician. 2015; 18:173–178.

10. Baron R, Janig W, With H. Sympathetic and afferent neurons projecting into forelimb and trunk nerves and the anatomical organization of the thoracic sympathetic outflow of the rat. J Auton Nerv Syst. 1995; 53:205–214.

11. Hogan QH, Erickson SJ, Haddox JD, Abram SE. The spread of solution during stellate ganglion block. Regional Anesthesia. 1992; 17:78–83.

12. Narouze S. Ultrasound-guided stellate ganglion block: safety and efficacy. Curr Pain Headache Rep. 2014; 18:424.

14. Maestroni GJ. Sympathetic nervous system influence on the innate immune response. Ann N Y Acad Sci. 2006; 1069:195–207.

15. Maestroni GJ. Modulation of skin norepinephrine turnover by allergen sensitization: Impact on contact hypersensitivity and T helper priming. J Invest Dermatol. 2004; 122:119–124.

16. Schlereth T, Birklein F. The sympathetic nervous system and pain. Neuromolecular Med. 2008; 10:141–147.

17. Westerhaus MJ, Loewy AD. Central representation of the sympathetic nervous system in the central cortex. Brain Res. 2001; 903:117–127.

18. Mulvaney SW, McLean B, de Leeuw J. The use of stellate ganglion block in the treatment of panic/anxiety symptoms with combat-related post-traumatic stress disorder; preliminary results of long-term follow-up: a case series. Pain Pract. 2010; 10:359–365.

19. Lynch ME, Elgeneidy AK. The role of sympathetic activity in neuropathic orofacial pain. J Orofac Pain. 1996; 10:297–305.

20. Lipov EG, Joshi JR, Sanders S, Slavin KV. A unifying theory linking the prolonged efficacy of the stellate ganglion block for the treatment of chronic regional pain syndrome (CRPS), hot flashes, and posttraumatic stress disorder (PTSD). Med Hypotheses. 2009; 72:657–661.
21. Salvaggio I, Adducci E, Dell'Aquila L, Rinaldi S, Marini M, Zappia L, et al. Facial pain: a possible therapy with stellate ganglion block. Pain Med. 2008; 9:958–962.
22. Matsuura M, Ando F, Sahashi K, Torii Y, Hirose H. The effect of stellate ganglion block on prolonged postoperative ocular pain. Nippon Ganka Gakkai Zasshi. 2003; 107:607–612.

23. Jeon YH, Kim JH. Treatment of Atypical Facial Pain with Stellate Ganglion Block. J Korean Dent Soc Anesthesiol. 2014; 14:173–175.

24. Jeon Y, Kim D. The effect of stellate ganglion block on the atypical facial pain. J Dent Anesth Pain Med. 2015; 15:35–37.

26. Wang QX, Wang XY, Fu NA, Liu JY, Yao SL. Stellate ganglion block inhibits formalin-induced nociceptive responses: mechanism of action. Eur J Anaesthesiol. 2005; 22:913–918.

27. Obermann M, Yoon MS, Esc D, Naschke M, Kaube H, Diener HC, et al. Impared trigeminal nociceptive processing in patients with trigeminal neuralgia. Neurology. 2007; 69:835–841.

28. Shanthanna H. Utility of stellate ganglion block in atypical facial pain: a case report and consideration of its possible mechanisms. Case Rep Med. 2013; 2013:293826.
29. Walega DR, Smith C, Epstein JB. Bilateral stellate ganglion blockade for recalcitrant oral pain from Burning Mouth Syndrome: a case report. J Oral Facial Pain Headache. 2014; 28:171–175.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download