Abstract
Purpose
Necrotizing enterocolitis and intestinal perforation are the most common surgical emergency in the neonatal intensive care unit. The purpose of this study is to evaluate if peritoneal drainage (PD) is beneficial in extremely low birth weight infants with intestinal perforation.
Methods
Retrospective cohort study of extremely low birth weight infants with a diagnosis of intestinal perforation. They were received primary PD (n = 23, PD group) or laparotomy (n = 13, LAP group). Laboratory and physiologic data were collected and organ failure scores calculated and compared between preprocedure and postprocedures. Data were analyzed using appropriated statistical tests.
Results
Between January 2005 and December 2015, 13 infants (male:female = 9:4) received laparotomy. Of 23 infants (male:female = 16:7) received PD, 20 infants received subsequent laparotomy. There were no demographic differences between PD and LAP groups. And there were no differences in total organ score in either group (PD, P = 0.486; LAP, P = 0.115). However, in LAP group, respiratory score was statistically improved between pre- and postprocedure organ failure score (P = 0.02). In physiologic parameter, PD group had a statistically worsening inotropics requirement (P = 0.025). On the other hand, LAP group had a improvement of PaO2/FiO2 ratio (P = 0.01).
Go to : 
Necrotizing enterocolitis (NEC) and intestinal perforation are the most common surgical emergencies in the neonatal intensive care unit [12]. With a prevalence of approximately 15%, they are especially common in extremely low birth weight (ELBW) infants, who are also the most likely to have high mortality and morbidity [3] and long-term consequences [3]. Although there have been advances in neonatal care in recent decades, NEC continues to challenge pediatric surgeons. Peritoneal drainage (PD) was first proposed as a treatment of intestinal perforation in ELBW infants in 1974 by Ein et al. [4]. Since then, PD has been used as both a definitive treatment and as a temporizing approach, although which is more efficient is debated.
Two randomized controlled trials have compared primary laparotomy with PD in low birth weight infants with intestinal perforation [56]. These trials did not show that PD improved survival or secondary outcomes. In addition, Rees et al. [7] suggested that PD is neither a safe alternative to laparotomy in ELBW infants with intestinal perforation nor an effective temporizing measure. However, for ELBW infants, surgeons hesitate or find it hard to choose laparotomy because laparotomy or general anesthesia itself can be fatal for such infants and because the patients may worsen during the long procedure preparation time [8].
The purpose of our present study was, first, to review the results of the management of ELBW infants with intestinal perforation in our center and, second, to evaluate if PD showed benefits as a temporizing approach in ELBW infants with intestinal perforation in a single center during the past 10 years.
Go to : 
We reviewed all cases of ELBW infants who underwent PD or laparotomy for intestinal perforation at Asan Medical Center, Seoul, Korea, from January 2005 to December 2015. We retrospectively collected data on gestational age, birth weight, Apgar score 1 and 5, type of ventilation, combined problems, clinical signs, and radiographic and laboratory findings. The study protocol was approved by the Institutional Review Board of Asan Medical Center (protocol number, S2019-1054-0001), and all study procedures complied with articles in the Declaration of Helsinki. Patient informed consent was waived because of the retrospective nature of the study. ELBW infants received primary PD (n = 23, PD group) or laparotomy (n = 13, LAP group). In the PD group, 20 patients underwent primary PD as a temporalizing approach followed by corrective laparotomy, whereas 3 patients underwent only PD.
PD was performed as a bedside procedure in the neonatal unit immediately upon detection of free air in the patient. The drain was inserted by the pediatric surgeon under sterile conditions using local anesthesia. An incision was made in the right or left lower quadrant of the abdomen and a drain was inserted. In the early period of the study, we decided whether the patient needed a further surgical procedure according to the general condition of the patient. If the patient was stable and the drainage contents were not dirty, we chose close observation without laparotomy. Between January 2005 and December 2008, four of 7 patients underwent laparotomy after PD. In the late period, we planned to perform laparotomy within 48 h regardless of the condition of the patient.
Laparotomy was performed either in an operating room or in the neonatal unit depending on the patient's condition. The choice of surgical procedure depended on the extent of the disease and the condition of the patient and the decision was made at the time of surgery by the surgeon. Two patients of 13 LAP group underwent primary anastomosis and 11 patients underwent enterostomy. In PD group, 5 patients underwent primary anastomosis without enterostomy and 14 patients underwent enterostomy operation. One patient who underwent laparotomy in 2011 got PD only due to severe adhesion.
Laboratory and physiological data were collected and organ failure scores were calculated and compared between the preprocedure and postprocedure periods. Physiological data, including heart rate, mean arterial pressure, inotrope requirement, type of ventilation, and arterial partial pressure of oxygen/fraction of inspired oxygen (PaO2/FiO2) ratio, were collected before and 24 hours after (usually, next day) treatment. Organ failure scores for cardiovascular, respiratory, hepatic, renal, and coagulation status were calculated using a modified sequential organ failure assessment score [9]. These scores were then compared between before and after the treatment (PD or LAP).
Data are expressed as the mean ± standard deviation if normally distributed or the median (range) if not and were analyzed using the Wilcoxon test or Mann-Whitney test as appropriate using SPSS ver. 17.0 (SPSS Inc, Chicago, IL, USA). A P-value < 0.05 was considered significant.
Go to : 
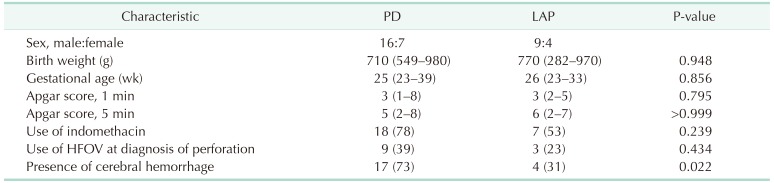
Of the 23 infants analyzed in this study (male:female = 16:7) who underwent PD, 20 underwent subsequent laparotomy and 3 underwent only PD. Thirteen infants (male:female = 9:4) underwent laparotomy alone. There were no demographic differences between the PD and LAP groups, including no difference in gestational age (25 weeks vs. 26 weeks, respectively; P = 0.856), birth weight (710 g [range, 549–980 g] vs. 770 g [range, 282–970 g], respectively; P = 0.948), Apgar score 1 (3 [range, 1–8] vs. 3 [range, 2–5], respectively; P = 0.795), and Apgar score 5 (5 [range, 2–8] vs. 6 [range, 2–7], respectively; P > 0.999) (Table 1). In the PD group, 22 patients needed a ventilator at birth; of these 22 infants, 9 required high-frequency oscillatory ventilation at the time of diagnosis of intestinal perforation. In the LAP group, 11 of the 13 patients needed a ventilator at birth and 3 patients received high-frequency oscillatory ventilation at diagnosis (P = 0.434) (Table 1). Seventeen patients had intracerebral hemorrhage, germinal matrix hemorrhage, or intraventricular hemorrhage from grade I to grade IV in the PD group. In contrast, only 4 patients had a brain hemorrhage in the LAP group (P = 0.022) (Table 1). Three of 13 patients in LAP group were expired and five of 23 patients in PD group. One patient in PD group was expired on the operating room table during enterotomy after PD. Another patient was expired due to postoperative shock and disseminated intravascularcoagulation one day after enterostomy.
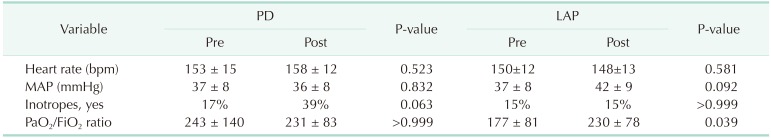
There were no significant differences between the preprocedural and postprocedural heart rate or mean arterial pressure in the PD or LAP groups (Table 2). Of the 23 patients in the PD group, 4 (17%) required preprocedural inotropes compared with 9 (39%) in the postprocedure period (P = 0.063). In the 13 infants in the LAP group, 2 (15%) required preoperative inotropes and 2 (15%) required postoperative inotropes (P > 0.999). Thus, the PD group had a worse inotropic requirement, although the difference was not significant.
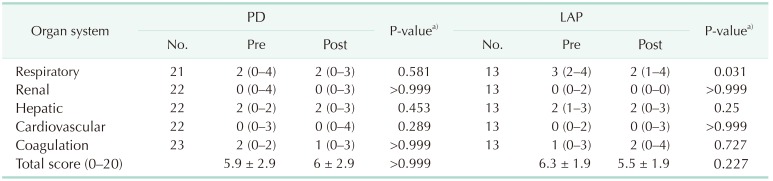
There was no significant difference in the PaO2/FiO2 ratio between the pre- and postprocedure periods in the PD group (P > 0.999) (Table 2). There was thus no significant alteration in the respiratory component of the organ failure score (preprocedure 2 [0–4] vs. postprocedure 2 [0–3], P = 0.581) (Table 3). On the other hand, there was a significant difference in the PaO2/FiO2 ratio between the pre- and postprocedure periods in the LAP group (P = 0.039), which resulted in a significant change in the organ failure score (preprocedure 3 [2–4] vs. postprocedure 2 [1–4]; P = 0.031).
There were no significant changes in the renal, hepatic, or coagulation status between the preprocedure and postprocedure periods in either the PD or LAP groups, as evidenced from the respective components of the organ failure score (Table 3). In addition, there were no differences in the total organ failure score in either group (PD, P > 0.999; LAP, P = 0.227). However, in the LAP group, the respiratory score significantly improved between the pre- and postprocedure organ failure scores (P = 0.031).
Go to : 
A 2002 survey of pediatric surgeons in the United Kingdom reported that PD was a widely used technique for the management of neonates with bowel perforation [10]. That study found that 58% used PD as a definitive treatment, 57% to stabilize neonates before transfer, and 95% to stabilize them before laparotomy. Ein et al. [4] reported that PD before laparotomy in NEC improved the general condition of the infants. Some subsequent reports suggested that PD helped to improve the general condition of patients, with further beneficial effects on morbidity and mortality [111213].
However, other trials found that PD did not improve survival in ELBW infants with intestinal perforation. A randomized controlled trial performed in the United States (NECSTEPS) showed no difference in survival at 90 days between infants managed with PD and laparotomy [5]. The NECSTEPS trial randomized infants less than 1,500 g with proven suspected perforation to PD or primary laparotomy but discouraged the use of early rescue laparotomy. Of the 55 infants included in their study, 5 (9%) required delayed laparotomy for clinical deterioration compared with 26 of the 35 infants (74%) included in this trial. The NET trial was another randomized controlled study performed in Europe [6]. This study also found that survival was not improved by the insertion of a drain as a temporizing method. Moreover, data from this trial showed that use of PD as a temporizing measure to stabilize patients in preparation for laparotomy is not an effective strategy [7].
In the past, we had considered 2 methods to be definitive treatments when we diagnosed ELBW infants with intestinal perforation [414]. All the patients who underwent laparotomy diagnosed as a NEC in this study. However, 3 patients without surgery could not be diagnosed with NEC because we did not know whether it was NEC or spontaneous intestinal perforation. The 3 current study patients in whom PD was used as a definitive procedure are currently still alive. There were no demographic and laboratory differences between these 3 cases and the other patients who underwent subsequent laparotomy. It is possible that we assumed that they had isolated intestinal perforation or early, localized NEC. However, based on the results of several studies, the policy of our center regarding the treatment of ELBW infants with intestinal perforation has changed and now PD is used as a temporizing method. We use PD to provide time when patients are too unstable for surgery or when the patient surroundings are not suitable for emergency surgery. Thus, we expected that PD would provide some benefits to unstable patients. Our current results have not shown an immediate improvement in physiologic status after PD. Rather inotrope requirement was increased after PD, although there was no significant difference between the groups and no improvement in the respiration parameters.
A major limitation of our present study was its retrospective uncontrolled design and our study has biases inherent to such a method; however, its purpose was to examine whether unstable ELBW infants with intestinal perforation benefited from PD. None of these infants had complications after PD, but they were not stabilized either. Our focus was on the bedside decision of whether to perform laparotomy or PD and, according to these results, patients are more likely to benefit when they undergo immediate laparotomy than PD. In addition, we take account of the fact that the infants in the group that underwent PD as a definitive treatment are all alive. It is difficult to determine whether PD is effective for some patients. However, if there are developments in the imaging techniques or laparoscopic surgical environment that can be applied to these unstable ELBW infants, PD might still be considered a useful method in selected infants in the future.
In conclusion, PD does not improve clinical status in ELBW infants with intestinal perforation. We therefore recommend the use of early laparotomy in infants with bowel perforation if the treating center is suitable for bedside operations and the operation can be started immediately. However, if no such situation is available, PD may still play a role in treating intestinal perforation in such infants, depending on the patient.
Go to : 
References
1. Santulli TV, Schullinger JN, Heird WC, Gongaware RD, Wigger J, Barlow B, et al. Acute necrotizing enterocolitis in infancy: a review of 64 cases. Pediatrics. 1975; 55:376–387. PMID: 1143976.

2. Kliegman RM, Fanaroff AA. Necrotizing enterocolitis. N Engl J Med. 1984; 310:1093–1103. PMID: 6369134.

3. Blakely ML, Lally KP, McDonald S, Brown RL, Barnhart DC, Ricketts RR, et al. Postoperative outcomes of extremely low birth-weight infants with necrotizing enterocolitis or isolated intestinal perforation: a prospective cohort study by the NICHD Neonatal Research Network. Ann Surg. 2005; 241:984–989. PMID: 15912048.
4. Ein SH, Marshall DG, Girvan D. Peritoneal drainage under local anesthesia for perforations from necrotizing enterocolitis. J Pediatr Surg. 1977; 12:963–967. PMID: 592076.

5. Moss RL, Dimmitt RA, Barnhart DC, Sylvester KG, Brown RL, Powell DM, et al. Laparotomy versus peritoneal drainage for necrotizing enterocolitis and perforation. N Engl J Med. 2006; 354:2225–2234. PMID: 16723614.

6. Rees CM, Eaton S, Kiely EM, Wade AM, McHugh K, Pierro A. Peritoneal drainage or laparotomy for neonatal bowel perforation? A randomized controlled trial. Ann Surg. 2008; 248:44–51. PMID: 18580206.
7. Rees CM, Eaton S, Khoo AK, Kiely EM, Pierro A. Members of NET Trial Group. Peritoneal drainage does not stabilize extremely low birth weight infants with perforated bowel: data from the NET Trial. J Pediatr Surg. 2010; 45:324–328. PMID: 20152345.

8. Kawakami A, Shirakawa Y, Shirahata A, Yano K, Morita M, Yasumoto K. Treatment of intestinal perforation in extremely low-birthweight infants. Pediatr Int. 2005; 47:404–408. PMID: 16091077.

9. Hall NJ, Eaton S, Peters MJ, Hiorns MP, Alexander N, Azzopardi DV, et al. Mild controlled hypothermia in preterm neonates with advanced necrotizing enterocolitis. Pediatrics. 2010; 125:e300–e308. PMID: 20100756.

10. Rees CM, Hall NJ, Eaton S, Pierro A. Surgical strategies for necrotising enterocolitis: a survey of practice in the United Kingdom. Arch Dis Child Fetal Neonatal Ed. 2005; 90:F152–F155. PMID: 15724040.

11. Ricketts RR, Jerles ML. Neonatal necrotizing enterocolitis: experience with 100 consecutive surgical patients. World J Surg. 1990; 14:600–605. PMID: 2238659.

12. Cheu HW, Sukarochana K, Lloyd DA. Peritoneal drainage for necrotizing enterocolitis. J Pediatr Surg. 1988; 23:557–561. PMID: 3418476.

13. Ein SH, Shandling B, Wesson D, Filler RM. A 13-year experience with peritoneal drainage under local anesthesia for necrotizing enterocolitis perforation. J Pediatr Surg. 1990; 25:1034–1036. PMID: 2262853.

14. Moss RL, Dimmitt RA, Henry MC, Geraghty N, Efron B. A meta-analysis of peritoneal drainage versus laparotomy for perforated necrotizing enterocolitis. J Pediatr Surg. 2001; 36:1210–1213. PMID: 11479858.

Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download