Abstract
Purpose
Unstable pelvic fracture with bleeding can be fatal, with a mortality rate of up to 40%. Therefore, early detection and treatment are important in unstable pelvic trauma. We investigated the early predictive factors for possible embolization in patients with hemodynamically unstable pelvic trauma.
Methods
From January 2011 to December 2013, 46 patients with shock arrived at a single hospital within 24 hours after injury. Of them, 44 patients underwent CT scan after initial resuscitation, except for 2 who were dead on arrival. Nine patients with other organ injuries were excluded. Seventeen patients underwent embolization. A single radiologist measured the width (longest length in axial view) and length (longest length in coronal view) of pelvic hematoma on CT scans. Demographic, clinical, and radiological data were reviewed retrospectively.
Results
Among 35 patients with hemodynamically unstable pelvic fracture, 22 (62.9%) were men. Width (P = 0.002) and length (P = 0.006) of hematoma on CT scans were significantly different between the embolization and nonembolization groups. The predictors of embolization were width of pelvic hematoma (odds ratio [OR], 1.07; P = 0.028) and female sex (OR, 10.83; P = 0.031). The cutoff value was 3.35 cm. More embolization was performed (OR, 12.00; P = 0.003) and higher mortality was observed in patients with hematoma width >3.35 cm (OR, 4.96; P = 0.048).
Go to : 
Patients with pelvic bone fractures still present a challenge to trauma surgeons despite the development of many diagnostic and therapeutic techniques. Many recent studies have reported that the overall mortality rates range from 18% to 40% [12345]. Although many treatment guidelines have been established and many treatment modalities have been improved, pelvic trauma still has a high mortality rate [67].
The main problem in patients with hemodynamic unstable pelvic fractures is active bleeding. Hemorrhage due to damage of pelvic organs and blood vessels is the main cause of death in these patients [38]. Excessive bleeding causes coagulopathy, which leads to a lethal triad and is a major cause of death. Therefore, early detection and control of pelvic hemorrhage is necessary [579]. A multidisciplinary approach is needed for resuscitation, control of bleeding, and management of pelvic bone injuries. Therefore, the treatment of pelvic fractures requires integrated treatment by a variety of physicians, including trauma surgeons, orthopedic surgeons, and interventional radiologists [6].
About 85% of pelvic hemorrhage cases are caused by bony structure destruction and venous bleeding, and 10%–15% of patients present with arterial bleeding [1261011]. Pelvic angiography is the method of choice for controlling arterial bleeding. In patients with hemodynamically unstable pelvic trauma, angiographic embolization has become an important part of treatment for bleeding control. Angiographic embolization is proven to be a safe and effective treatment method in patients with pelvic trauma [12]. For the treatment of arterial injury in pelvic fracture, aggressive angiographic embolization has a high success rate [1456121314]. Nevertheless, although many studies have reported on the indications for pelvic angiography, precise guidelines have yet to be established. Many investigations have been carried out to determine the clinical predictors according to clinical or radiologic findings such as contrast extravasation on CT or fracture pattern on radiography [110141516]. However, there are still controversies in determining the need for angiography and embolization [21718].
The purpose of this study was to predict the necessity of embolization and the timing of angiography using CT scans.
Go to : 
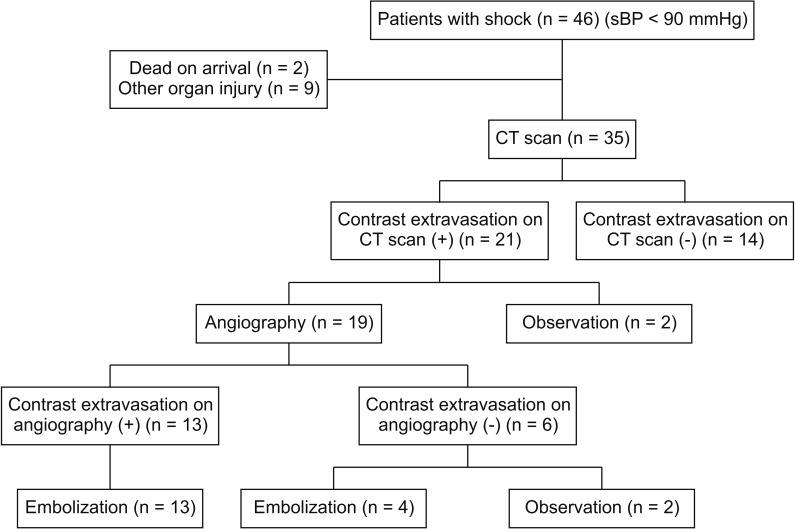
Among 120 patients with major trauma and pelvic bone fracture, 46 patients with shock (systolic blood pressure < 90 mmHg) arrived at a single hospital within 24 hours after injury from January 2011 to December 2013. Among these patients, 2 were declared dead on arrival. A total of 44 patients underwent CT after initial resuscitation. Nine patients with other organ injuries were also excluded. A total of 21 patients had contrast extravasation on the CT scan, and 2 of them had stable vital signs and did not undergo angiography. Nineteen patients underwent angiography performed by specialized interventional radiologists. Among these patients, 13 underwent embolization because of contrast extravasation on angiography. On the other hand, 6 patients had no contrast extravasation on angiography. However, 4 patients underwent embolization owing to the possibility of arterial injury according to the judgment of the interventional radiologist, considering the vital signs and imaging findings at the time of the procedure. As a result, a total of 17 patients underwent embolization (Fig. 1).
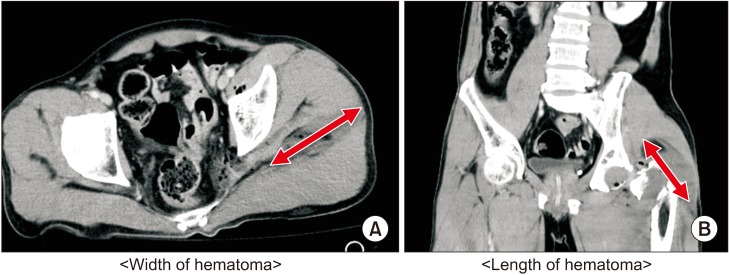
A single radiologist measured the width and length of the pelvic hematoma on CT scans (Fig. 2). We defined the width of hematoma as the longest length in the axial view of the CT scan and the length of hematoma as the longest length in the coronal view.
All medical records and radiographic findings were reviewed retrospectively. Demographic data and clinical information were collected from electronic medical records.
The Mann-Whitney U-test and Student t-test were used for comparing continuous variables. Multiple-group comparisons were performed using the Kruskal-Wallis test. A logistic stepwise regression analysis was performed to predict the need for possible embolization based on clinical variables and radiologic findings. The significance level was set at P < 0.05. Adjusted odds ratios and 95% confidence intervals were derived. Statistical analyses were conducted with the R ver. 3.3.2 (R Foundation for Statistical Computing, Vienna, Austria; http://www.R-project.org).
The study was approved by the Institutional Review Board (IRB) of Asan Medical Center (IRB No. 2018-0212). This study was conducted with a waiver of consent. This trial is registered with ClinicalTrials.gov (NCT03519594).
Go to : 
Among 35 patients with hemodynamically unstable pelvic fracture, 22 (62.9%) were men and 13 (37.1%) were women. Their ages at the time of injury ranged from 17 to 86 years, with a mean of 54.51 years. There were 21 patients (60.0%) who had traffic accidents. The mean time of arrival after the accident was 2.85 hours. A total of 10 patients died. Therefore, the mortality rate in our study was 28.6%. The mean time of CT scan examination was 98.49 minutes. At the time of admission, the mean systolic blood pressure was 69.00 ± 14.01 mmHg, which means the patients were in a state of shock. The median Glasgow Coma Scale (GCS) score and Injury Severity Score (ISS) were 14.0 (8.5–15.0) and 25.0 (22.0–40.5), respectively. Additionally, the mean lactate and hemoglobin levels were 5.97 ± 3.97 mmol/L and 10.63 ± 2.96 g/dL, respectively. The median red blood cell (RBC) transfusion volume was 8.0 (5.0–17.5) packs (Table 1).
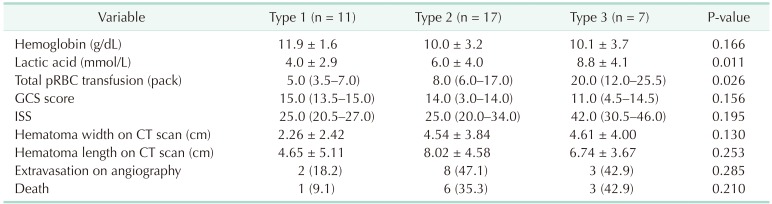
We divided the patients into 3 groups according to radiographic findings based on Tile's classification and compared each clinical variable (Table 2). There were no significant differences in other variables except for the amount of total packed RBC (pRBC) transfusion and lactate levels. As the severity of fracture patterns worsened, the amount of pRBC transfusion increased (P = 0.026). However, there was no correlation with extravasation on angiography or increased mortality according to fracture patterns.
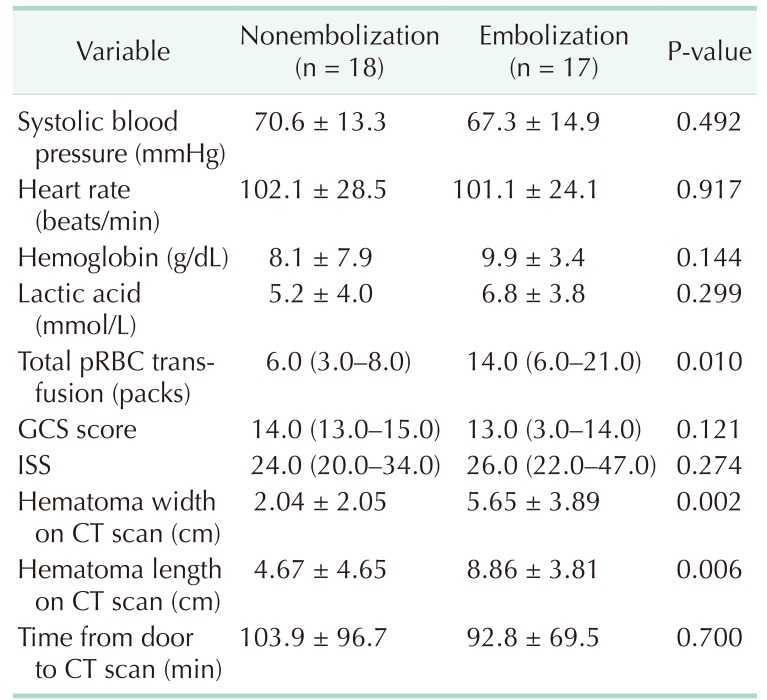
We compared the clinical variables of patients who underwent embolization and those who did not (Table 3). There were significant differences in width (2.04 ± 2.05 vs. 5.65 ± 3.89, P = 0.002) and length (4.67 ± 4.65 vs. 8.86 ± 3.81, P = 0.006) of hematoma on the CT scan on admission and in the amount of pRBC transfusion (6.0 [3.0–8.0] vs. 14.0 [6.0–21.0], P = 0.010) between the 2 groups. There was no significant difference in the CT scan time between the 2 groups (103.9 ± 96.7 vs. 92.8 ± 69.5, P = 0.700).
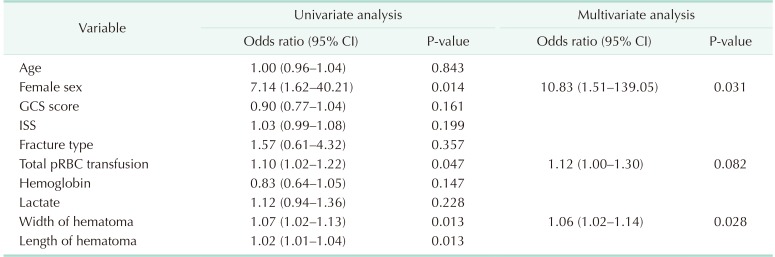
The predictors for embolization were estimated by substituting the following variables, such as GCS, ISS score, fracture type, amount of pRBC transfusion, hemoglobin level, lactate level, pelvic hematoma width, and pelvic hematoma length (Table 4). Moreover, the width of hematoma (OR, 1.06; 95% CI, 1.02–1.14; P = 0.028) and female sex (OR, 10.83; 95% CI, 1.51–139.05; P = 0.031) were the only significant variables.
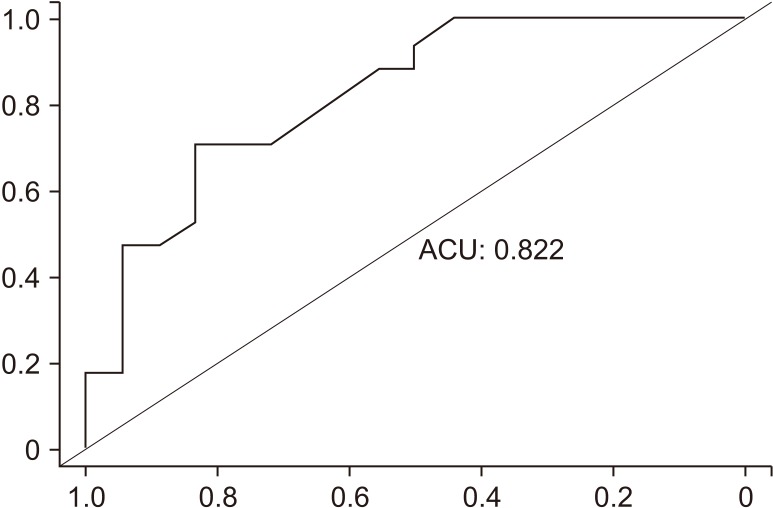
The cutoff value was obtained using the receiver operating characteristic curve for pelvic hematoma width (Fig. 3). The cutoff value was 3.35 cm (Table 5).
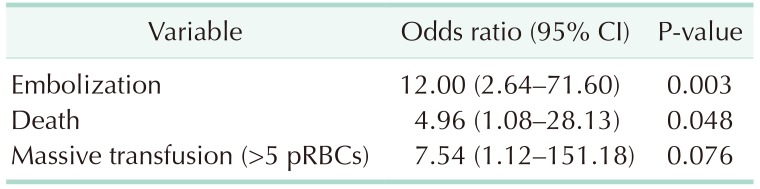
We also compared the incidence of angiographic embolization and massive transfusion and the mortality rate. The group with pelvic hematoma greater than 3.35 cm had a 12-fold higher probability of embolization than the other groups (OR, 12.00; 95% CI, 2.64–71.60; P = 0.003). Furthermore, the mortality rate was increased in the group with hematoma greater than 3.35 cm (OR, 4.96; 95% CI,1.08–28.13; P = 0.048). There was no significant difference in massive transfusion over 5 packs (OR, 7.54; 95% CI, 1.12–151.18; P = 0.076) (Table 6).
Go to : 
Early and aggressive angiographic intervention in patients with hemodynamically unstable pelvic fractures can significantly improve survival. Generally, the reported indications for angiographic intervention include hemodynamic instability, specific pelvic fracture patterns, and extravasation or large pelvic hematoma on the CT scan [71319202122]. Recently, many studies have reported the importance of appropriate patient selection in therapeutic angiographic embolization.
As mentioned earlier, many studies have reported that the prognosis of patients with unstable pelvic fracture can be predicted according to the fracture pattern [1121314151623]. However, in our study, as the severity of pelvic fractures increased, the amount of pRBC transfusion also increased, but the incidence of arterial hemorrhage or the mortality rate did not increase. These results show that there is a limit to setting the treatment plan based on radiographic findings only. Owing to the development of whole-body CT, CT scans can now be taken immediately after the initial resuscitation in patients with major trauma. CT scans can confirm the severity of pelvic fracture and any coexisting injury to other organs [424]. In our study, although all patients were hemodynamically unstable, they underwent CT after admission. Upon stabilization of vital signs to some extent, a whole-body CT scan should be taken as soon as possible to help make treatment decisions [31124].
Many studies have been conducted to determine whether the need for therapeutic angiography and embolization could be predicted using CT findings, such as contrast blush or a large hematoma. However, there have been many controversies about making therapeutic decisions based on those CT findings. Blackmore et al. [21] measured the volume of pelvic hematoma by using a special software called ImageJ, and found that the outcome could be predicted using the hematoma size. Our study also showed that if the hematoma width is greater than 3.35 cm on the CT scan, embolization should be performed. However, it should be noted that Blackmore et al. [21] used special software and it is difficult to apply such measurements in an emergency situation. In our study, we could easily measure the hematoma width by using the longest length in the axial view of CT scan, and it was useful enough to predict outcome easily in an emergency situation. Dreizin and Bodanapally [25] reported a comparative study of our longestdiameter measurement method and a manual segmentation method in which hematoma volume is measured through semiautomated segmentation. Our method showed a higher correlation with the semiautomated segmentation method than with other methods. However, it is also difficult to have this measurement software in all hospitals, and there may be limitations in using this software in emergency situations.
Brown et al. [22] recommended immediate angiography in patients with a large pelvic hematoma or contrast extravasation on the CT scan. Moreover, they reported that active bleeding could not be ruled out even if there was no extravasation on the CT scan. Because hypotension or arterial spasm can cause contrast extravasation not to appear on CT scans, it cannot be assumed that there is no active bleeding in the absence of extravasation. In our study, when the pelvic hematoma width was greater than 3.35 cm, angiographic embolization was performed more frequently and the mortality rate also increased. Therefore, more aggressive angiographic embolization is recommended if the pelvic hematoma size measured on the CT scan is greater than 3.35 cm.
Furthermore, in our study, angiographic embolization was more frequently done in female patients. The reason for the high incidence of embolization in female patients is that the pelvis is wider in women than in men, and hemostasis by pressure may not be sufficient. Some studies have also reported female sex as a predictor for angiography [13].
In our study, there was no angiographic extravasation in 6 patients and 4 of them underwent prophylactic embolization. The vital signs at the time of the procedure were unstable and intrapelvic hemorrhage was suspected; thus, prophylactic embolization was performed and the vital signs improved thereafter. Embolization was mainly performed in the internal iliac artery, which is located near the fracture site. Hymel et al. [2] reported that prophylactic embolization despite negative angiograms was helpful in hemorrhage control for patients who are being actively transfused. In our study, similar results were obtained, and patients who underwent prophylactic embolization were included in the embolization group.
This study has several limitations. First, this study has the inherent limitations of retrospective studies. At the time of the study, angiography was done according to the patient's vital signs or the trauma surgeon's judgment at that time rather than the size of the pelvic hematoma, and these may have affected the classification of the embolization group. For more accurate results, prospective studies should be conducted to compare the clinical outcomes of the embolization group according to the hematoma width measured at the time of admission. Second, there is a limit to the interobserver correlation because a single radiologist measured the width of the pelvic hematoma while reviewing the CT findings. As a rough measurement of the longest diameter on the CT scan was done, there may be differences depending on the observer. It is necessary to perform additional studies to compare interobserver correlation in the existence of 2 or more observers.
In conclusion, hemodynamically unstable pelvic fracture remains a significant challenge in providing appropriate treatment. The safety and efficacy of angiographic embolization in patients with unstable pelvic fracture has already been proven. Therefore, angiographic embolization is recommended depending on the patient's condition. Moreover, the trauma surgeon's judgment is an important factor in the treatment. With the advances in CT, even if the patient's vital signs are somewhat unstable, rapid examination is possible and the severity of pelvic fracture can be evaluated. CT is useful for the initial identification of the need for embolization. The width of pelvic hematoma can predict possible embolization. If the width of pelvic hematoma on the CT scan is greater than 3.35 cm, more aggressive embolization should be considered.
Go to : 
References
1. El-Haj M, Bloom A, Mosheiff R, Liebergall M, Weil YA. Outcome of angiographic embolisation for unstable pelvic ring injuries: factors predicting success. Injury. 2013; 44:1750–1755. PMID: 23796438.

2. Hymel A, Asturias S, Zhao F, Bliss R, Moran T, Marshall RH, et al. Selective versus nonselective embolization versus no embolization in pelvic trauma: a multicenter retrospective cohort study. J Trauma Acute Care Surg. 2017; 83:361–367. PMID: 28463936.
3. Tanizaki S, Maeda S, Matano H, Sera M, Nagai H, Ishida H. Time to pelvic embolization for hemodynamically unstable pelvic fractures may affect the survival for delays up to 60 min. Injury. 2014; 45:738–741. PMID: 24314873.
4. Tesoriero RB, Bruns BR, Narayan M, Dubose J, Guliani SS, Brenner ML, et al. Angiographic embolization for hemorrhage following pelvic fracture: is it “time” for a paradigm shift? J Trauma Acute Care Surg. 2017; 82:18–26. PMID: 27602911.
5. White CE, Hsu JR, Holcomb JB. Haemodynamically unstable pelvic fractures. Injury. 2009; 40:1023–1030. PMID: 19371871.

6. Coccolini F, Stahel PF, Montori G, Biffl W, Horer TM, Catena F, et al. Pelvic trauma: WSES classification and guidelines. World J Emerg Surg. 2017; 12:5. PMID: 28115984.

7. Cullinane DC, Schiller HJ, Zielinski MD, Bilaniuk JW, Collier BR, Como J, et al. Eastern Association for the Surgery of Trauma practice management guidelines for hemorrhage in pelvic fracture--update and systematic review. J Trauma. 2011; 71:1850–1868. PMID: 22182895.

8. Dalal SA, Burgess AR, Siegel JH, Young JW, Brumback RJ, Poka A, et al. Pelvic fracture in multiple trauma: classification by mechanism is key to pattern of organ injury, resuscitative requirements, and outcome. J Trauma. 1989; 29:981–1000. PMID: 2746708.
9. Smith W, Williams A, Agudelo J, Shannon M, Morgan S, Stahel P, et al. Early predictors of mortality in hemodynamically unstable pelvis fractures. J Orthop Trauma. 2007; 21:31–37. PMID: 17211266.

10. Yoon W, Kim JK, Jeong YY, Seo JJ, Park JG, Kang HK. Pelvic arterial hemorrhage in patients with pelvic fractures: detection with contrast-enhanced CT. Radiographics. 2004; 24:1591–1605. PMID: 15537967.

11. Brun J, Guillot S, Bouzat P, Broux C, Thony F, Genty C, et al. Detecting active pelvic arterial haemorrhage on admission following serious pelvic fracture in multiple trauma patients. Injury. 2014; 45:101–106. PMID: 23845571.

12. Velmahos GC, Toutouzas KG, Vassiliu P, Sarkisyan G, Chan LS, Hanks SH, et al. A prospective study on the safety and efficacy of angiographic embolization for pelvic and visceral injuries. J Trauma. 2002; 53:303–308. PMID: 12169938.

13. Salim A, Teixeira PG, DuBose J, Ottochian M, Inaba K, Margulies DR, et al. Predictors of positive angiography in pelvic fractures: a prospective study. J Am Coll Surg. 2008; 207:656–662. PMID: 18954776.

14. Anandakumar V, Hussein FK, Varuun B, Zhu R. Predictive parameters for angiography and embolization in the bleeding pelvic fracture. J Clin Orthop Trauma. 2013; 4:70–74. PMID: 26403627.

15. Demetriades D, Karaiskakis M, Toutouzas K, Alo K, Velmahos G, Chan L. Pelvic fractures: epidemiology and predictors of associated abdominal injuries and outcomes. J Am Coll Surg. 2002; 195:1–10. PMID: 12113532.
16. Blackmore CC, Cummings P, Jurkovich GJ, Linnau KF, Hoffer EK, Rivara FP. Predicting major hemorrhage in patients with pelvic fracture. J Trauma. 2006; 61:346–352. PMID: 16917449.

17. Suzuki T, Smith WR, Moore EE. Pelvic packing or angiography: competitive or complementary? Injury. 2009; 40:343–353. PMID: 19278678.

18. Burlew CC, Moore EE, Stahel PF, Geddes AE, Wagenaar AE, Pieracci FM, et al. Preperitoneal pelvic packing reduces mortality in patients with life-threatening hemorrhage due to unstable pelvic fractures. J Trauma Acute Care Surg. 2017; 82:233–242. PMID: 27893645.

19. Pereira SJ, O'Brien DP, Luchette FA, Choe KA, Lim E, Davis K Jr, et al. Dynamic helical computed tomography scan accurately detects hemorrhage in patients with pelvic fracture. Surgery. 2000; 128:678–685. PMID: 11015102.

20. Eastridge BJ, Starr A, Minei JP, O'Keefe GE, Scalea TM. The importance of fracture pattern in guiding therapeutic decision-making in patients with hemorrhagic shock and pelvic ring disruptions. J Trauma. 2002; 53:446–450. PMID: 12352479.

21. Blackmore CC, Jurkovich GJ, Linnau KF, Cummings P, Hoffer EK, Rivara FP. Assessment of volume of hemorrhage and outcome from pelvic fracture. Arch Surg. 2003; 138:504–508. PMID: 12742953.

22. Brown CV, Kasotakis G, Wilcox A, Rhee P, Salim A, Demetriades D. Does pelvic hematoma on admission computed tomography predict active bleeding at angiography for pelvic fracture? Am Surg. 2005; 71:759–762. PMID: 16468513.

23. Holstein JH, Culemann U, Pohlemann T. Working Group Mortality in Pelvic Fracture Patients. What are predictors of mortality in patients with pelvic fractures? Clin Orthop Relat Res. 2012; 470:2090–2097. PMID: 22354608.

24. Huber-Wagner S, Biberthaler P, Haberle S, Wierer M, Dobritz M, Rummeny E, et al. Whole-body CT in haemodynamically unstable severely injured patients--a retrospective, multicentre study. PLoS One. 2013; 8:e68880. PMID: 23894365.
25. Dreizin D, Bodanapally UK, Neerchal N, Tirada N, Patlas M, Herskovits E. Volumetric analysis of pelvic hematomas after blunt trauma using semi-automated seeded region growing segmentation: a method validation study. Abdom Radiol (NY). 2016; 41:2203–2208. PMID: 27349420.

Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download