Abstract
Purpose
Radical lymph node dissection for right-sided colon cancer is technically challenging. No clear guideline is available for surgical resection of clinical stage I right-sided colon cancer. This study was designed to review the pathologic stage of clinical stage I right-sided colon cancer and determine the relevant extent of surgical resection.
Methods
Patients were treated for clinical stage I right-sided colon cancers (cecal, ascending, hepatic flexure, and proximal transverse colon) between July 2006 and December 2014 at a tertiary teaching hospital. Open surgery was not included because laparoscopic surgery is an initial major procedure in the institution.
Results
During the study period, 80 patients diagnosed with clinical stage I right-sided colon cancer were classified into 2 groups according to the pathology: stage 0/I and II/III. Tumor sizes were larger in the stage II/III group (P = 0.003). The stage II/III group had higher rates of vascular (P = 0.023) and lymphatic invasion (P = 0.023) and lower rates of well differentiation (P = 0.022). During follow-up, 1 case of local and 4 cases of systemic recurrences were found. Multivariate analysis to confirm odds ratios affecting change from clinical stage I to pathological stage II/III showed that tumor size (P = 0.010) and the number of retrieved lymph nodes (P = 0.046) were risk factors.
Go to : 
Complete mesocolic excision (CME) in the mesocolic plane with central vascular ligation (CVL), or high vascular ties, has become widely recognized as the standard surgical technique for right-sided colon cancer since it was announced [1]. However, radical lymph node dissection for right-sided colon cancer remains technically challenging. Clinically, it is difficult to decide on radical lymphadenectomy in early stages.
In patients in surgically curable stages, there have been reports of cases where local or distant recurrence was found, despite surgical extents considered appropriate. Recently released in Japan, the guidelines for early-stage colon cancer, which are based on several studies [234], showed about 10% lymph node metastasis for pathological T1 (submucosa) cancers [5]. The National Comprehensive Cancer Network (NCCN) does not provide definite surgical treatment guidelines for early colorectal cancer [6]. The guidelines of the European Society for Medical Oncology (ESMO) only described wide surgical resection and anastomosis for stage I colon cancer [7].
Therefore, this study was designed to review the pathologic stage of the clinical stage I right-sided colon cancer, and to determine the relevant extent of surgical resection.
Go to : 
This was a retrospective study that analyzed prospective data from a database of patients with malignant colonic diseases. Patients were treated for clinical stage I right-sided colon cancers (cecal, ascending, hepatic flexure, and proximal transverse colon) between July 2006 and December 2014 at a tertiary teaching hospital. Clinical stage I is defined as a case which shows clinical T1 or T2 in preoperative abdominal CT and reads as negative lymph node metastasis. Open surgery was not included because laparoscopic surgery is an initial approaching procedure in the institution. All procedures were performed for the purpose of margin-negative (R0) resection because of the early clinical stage. The authors performed radical lymph node dissection when the principal nodes were dissected according to the 2014 Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines for the treatment of colorectal cancer [8], which included the roots of lymph nodes at ileocolic (203), right colic (213) if present, and middle colic (223) arteries.
Data from patients included age, sex, body mass index (BMI), American Society of Anesthesiologists (ASA) physical status classification, past medical history including previous abdominal surgery, and preoperative CEA level. Postoperative parameters included tumor location, resected extent, operative duration excluding the initial anesthetic duration, and estimated blood loss (EBL). Pathological outcomes included tumor size and margins, number of retrieved and pathologic lymph nodes, invasion, and differentiation. Postoperative outcomes such as postoperative CEA, days to bowel movement, time to flatus and defecation, diet resumption, length of hospital stay, short-term complications, and postoperative mortality were compared. Oncological outcomes included locoregional recurrence and distant metastasis during a 5-year follow-up period.
Staging was based on the eighth edition of the tumor, node and metastasis (TNM) classification of colon cancer proposed by the Union for International Cancer Control (UICC) and the American Joint Committee on Cancer; cases included before 2010 were restaged. The patients visited the hospital quarterly for the first 2 years and semiannually in the last 3 years. During follow-up, serum CEA analyses, chest and abdominopelvic CT were performed semiannually, whereas total colonoscopy was performed annually. Recurrence was defined as a pathologically identified or radiologically proven local or systemic metastasis. The study was approved by the Institutional Review Board of Chonnam National University Hospital (CNUH-2019-119), and the need for written informed consent was waived because of the retrospective nature of the study.
The 2 groups were compared using Student t-test or Mann–Whitney U-test for continuous data and chi-square or Fisher exact test for categorical data. For multivariate analyses, the authors fit binary logistic regression models to identify significant risk factors from a set of significant main effect variables (P < 0.15). Statistical analyses were performed using IBM SPSS Statistics ver. 20.0 (IBM Co., Armonk, NY, USA). A P-value ≤ 0.05 was considered statistically significant.
Go to : 
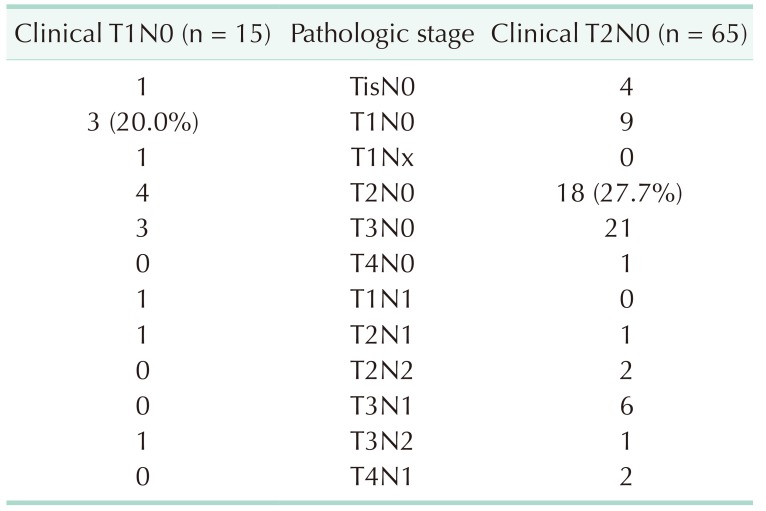
During the period, a total of 80 patients were diagnosed with clinical stage I right-sided colon cancer. There were 15 and 65 patients in clinical T1 and T2, respectively. Pathological stage was identified as 5, 35, 25, and 15 patients for stage 0, I, II, and III, respectively. Only 43.8% of the patients were diagnosed with pathological stage I. It was lower that clinical T stage was proved exactly to be pathological T stage: 20% (3 of 15) of T1N0 and 27.7% (18 of 65) of T2N0 (Table 1). According to the T and N stages, patients were classified into 2 groups – stage 0/I and stage II/III.
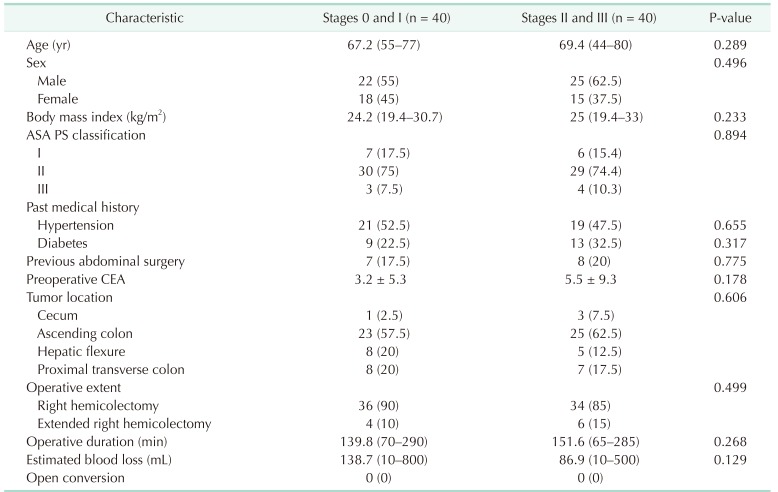
There was no difference between the 2 groups in age, sex, BMI, ASA physical status classification, past medical history, previous abdominal surgery, preoperative CEA, operative duration, and EBL. Ascending colon cancer was most prevalent in both groups, however, no difference in tumor location between the groups existed. Right hemicolectomy was performed in 87.5% cases, whereas extended right hemicolectomy was performed in the remaining cases. No open conversion case was recorded (Table 2).
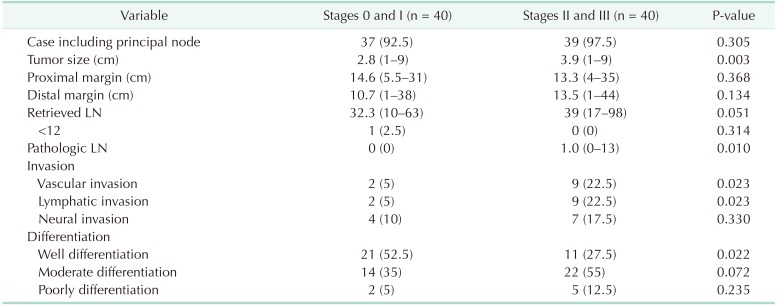
In all, it was confirmed in 95% of cases that principal nodes were 203 and/or 223, which were considered as radical lymph node dissection. For the size of tumors, an average of 2.8 cm for stage 0/I and 3.9 cm for stage II/III (P = 0.003) were recorded. The length of specimens' margins did not differ between the groups; the number of harvested lymph nodes (32.3 in stage 0/I and 39 in stage II/III) also showed no statistical difference (P = 0.051). Staging was not possible in only 1 patient with 10 retrieved lymph nodes. Tumors had higher frequencies in vascular and lymphatic invasion among patients in the stage II/III group (P = 0.023), whereas well differentiation was more frequent in stage 0/I (P = 0.022). About 25% of patients advanced to stage IIA and was classified as high-risk group (Table 3).
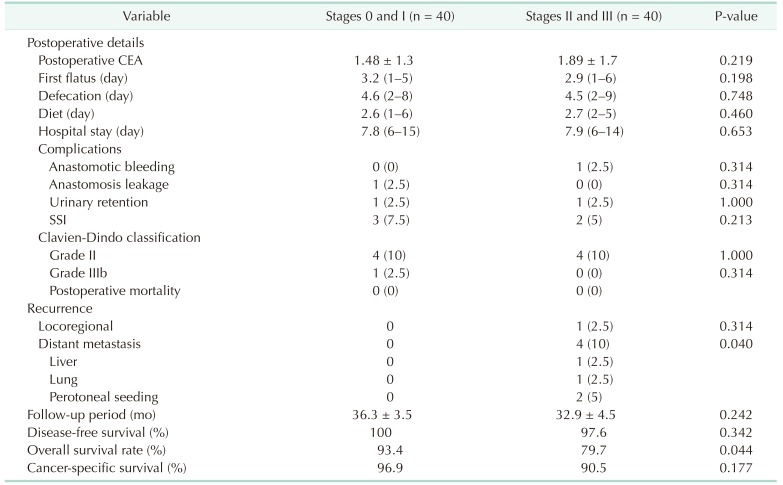
Postoperative CEA levels as before the surgery, first flatus, and defecation (indication of bowel recovery) were not different between the 2 groups. There also had no difference in postoperative defecation and the length of hospital stay. With no difference between both groups, 9 cases of postoperative complications were reported in total: surgical site infection (6.25%), urinary retention (2.5%), anastomosis bleeding (1 case), anastomosis leakage (1 case). No death was recorded within 30 days of the operations (Table 4).
During a median follow-up period of 34 months (36 and 33 months for stage 0/I and stage II/III groups, respectively), 1 local and 4 systemic recurrences were found in stage II/III group only; whereas distant metastasis occurred with significant difference between both groups (P = 0.040). A total of 6 cases were related cancer-specific death. There was no significant difference in relation with disease-free and cancer-specific survival although there was a difference between the groups with overall survival (Table 4).
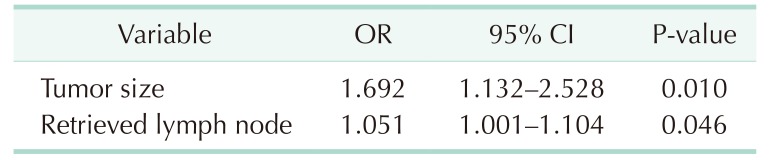
With logistic regression by backward elimination method, stage migration was found to be higher when it had larger tumor size (odds ratio [OR], 1.692; 95% confidence interval [CI]. 1.132–2.528; P = 0.010) and more retrieved lymph nodes (OR, 1.051; 95% CI, 1.001–1.104; P = 0.046) (Table 5).
Go to : 
Radical resection in colorectal cancer is now recognized as a standard surgical procedure. This is attributed to better lymph node yield, precise staging, lower local recurrence rates, and improved survival rates. However, the procedure in early cancers is not universally accepted because there has no guideline on whether radical resection is necessary. Neither the NCCN nor ESMO guidelines have clear definitions for the extent of lymphadenectomy in clinic stage I right colon cancer. This study was designed to determine the proper surgical range of clinical stage I right-sided colon cancer by comparison with the pathological stages of 80 patients. The results revealed that less than 50% of stage I cases were confirmed before and after surgery (43.8%, 35 of 80). In addition, larger tumor sizes and more harvested lymph nodes caused the migration to stage II/III. However, preoperative evaluation such as routine abdominal CT does not allow accurate evaluation for the extent of surgery. Therefore, early right-sided colon cancers need radical resection including lymph node dissection, just as needed in advanced cancers. Since no clear guidelines for clinical stage I colon cancer exist, the results of this study may prove significant in informing the formation of such guidelines.
According to a study by Hohenberger et al. [9], of the 1,438 colon cancer patients, 8.7% and 13.2% were in the pT1 and pT2 groups, respectively. Analysis of the 5-year locoregional recurrence showed 0.4% for stage I, but 0.9 and 1.2 for pT1 and pT2, respectively; although no statistical analysis had been performed, the recurrence of pT1/pT2 was higher than that of stage I. The guidelines for early colon cancers were recently mentioned in the JSCCR 2016 guidelines for the treatment of colorectal cancer [5]. The guidelines recommend D2 dissection, including intermediate lymph node, for both cT1 (submucosa) and cT2 (muscularis propria) cancers which show about 10% and 1% lymph node metastasis, respectively. However, due to insufficient evidence, no clear guidelines have been confirmed for D3 dissection, which also applies to some cT2 cancers.
Lymph node metastasis in early-stage colon cancers, which was also mentioned in JSCCR, has been studied severally. In pT1, nodal metastasis has been reported to be about 10% to 14.5%, and in pT2, it has been reported to be about 23.9% [23410]. Over 1 mm of submucosal invasion, lymphovascular invasion, poor differentiation, and tumor budding were reported as risk factors for lymphatic metastasis [11]. However, it is still difficult to determine the extent of the operation in advance, because pathological reports related to tumor depth, infusion, and differentiation cannot be verified preoperatively.
In a 2010 study on 342 colorectal cancer patients, a wide range of lymph node dissections improved long-term survival. The study compared the groups of D1 (simple) and D2 (extended) dissection using JSCCR guidelines [12]. Although the analysis was not conducted in stage I only, stages I and II showed that D2 dissection was one factor for better survival (P = 0.023) [13]. Reports on studies on the extent of resection, which cover right-sided colon cancer only, have quite recently been published. Using a cohort of 189 consecutive patients diagnosed with stages I–II colon cancer in 2014, a Norwegian study compared CME, having high (apical) vascular tie (D3 resection), with the conventional (standard, D2) approach. The CME group had a higher 3-year overall, disease-free, and cancer-specific survival [14]. The comparison between the group of classic right hemicolectomy and that of CME with CVL in the last 5 years also revealed a difference in recurrence rate (no recurrence in CME group vs. 21% of the standard group, P = 0.03) [15]. In a retrospective population-based study which included UICC stage I only, 4-year disease-free survival rates of CME and non-CME groups were 100% and 89.8%, respectively (P = 0.046). Cox regression confirmed CME as a significant independent predictive factor (hazard ratio, 0.59; 95% CI, 0.42–0.83) that increased disease-free survival. Median number of resected lymph nodes were 34 and 19 in CME and non-CME group (P < 0.0001), and specimen with 12 or more lymph nodes were 99% and 89% (P < 0.0001), respectively. The results showed that CME had an impact on the staging accuracy and determination of adjuvant treatment [16].
Routine application of CME can cause longer operating time, major vascular injury, and automatic nerve damage [17]. In response to this debate, an expert consensus on CME was recently published. Nine out of 13 experts responded to the question “Are there specific indications for CME? (Should it be performed in all patients; is it necessary for all stages?)”. Of the 9, 4 adapted CME to all stages, whereas the other 5 did not. Three out of the 5 respondents said that CME does not apply to stage I [18].
We note that this study had several limitations. Our interpretation of the results was limited by the small sample size. This retrospective design may also have led to selection bias despite the use of prospectively collected data. However, we expect that the present study will support well-designed, randomized clinical trials (RCTs) of colonic resection for clinical stage I right-sided colon cancer. In particular, we expect that the results of an RCT in comparing laparoscopic D2 dissection and CME [19], T-REX study [20], International Prospective Observational Cohort Study for Optimal Bowel Resection Extent and Central Radicality, to confirm a distribution of metastatic lymph nodes and prognostic outcomes according to the length of bowel resection, will reveal a suitable surgical extent for clinical stage I right-sided colon cancer.
In conclusion, radical lymph node dissection should be performed for exact staging with sufficient number of lymph nodes for right-sided colon cancer including clinical stage I. This will help determine appropriate adjuvant treatment and enhance survival rates, especially in large tumor sizes.
Go to : 
References
1. West NP, Hohenberger W, Weber K, Perrakis A, Finan PJ, Quirke P. Complete mesocolic excision with central vascular ligation produces an oncologically superior specimen compared with standard surgery for carcinoma of the colon. J Clin Oncol. 2010; 28:272–278. PMID: 19949013.

2. Kawamura YJ, Sakuragi M, Togashi K, Okada M, Nagai H, Konishi F. Distribution of lymph node metastasis in T1 sigmoid colon carcinoma: should we ligate the inferior mesenteric artery? Scand J Gastroenterol. 2005; 40:858–861. PMID: 16109663.

3. Choi PW, Yu CS, Jang SJ, Jung SH, Kim HC, Kim JC. Risk factors for lymph node metastasis in submucosal invasive colorectal cancer. World J Surg. 2008; 32:2089–2094. PMID: 18553050.

4. Huh JW, Kim HR, Kim YJ. Lymphovascular or perineural invasion may predict lymph node metastasis in patients with T1 and T2 colorectal cancer. J Gastrointest Surg. 2010; 14:1074–1080. PMID: 20431977.

5. Watanabe T, Muro K, Ajioka Y, Hashiguchi Y, Ito Y, Saito Y, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2016 for the treatment of colorectal cancer. Int J Clin Oncol. 2018; 23:1–34. PMID: 28349281.

6. Benson AB, Venook AP, Al-Hawary MM, Cederquist L, Chen YJ, Ciombor KK, et al. NCCN guidelines insights: colon cancer, version 2.2018. J Natl Compr Canc Netw. 2018; 16:359–369. PMID: 29632055.

7. Labianca R, Nordlinger B, Beretta GD, Mosconi S, Mandala M, Cervantes A, et al. Early colon cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013; 24 Suppl 6:vi64–vi72. PMID: 24078664.

8. Watanabe T, Itabashi M, Shimada Y, Tanaka S, Ito Y, Ajioka Y, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2014 for treatment of colorectal cancer. Int J Clin Oncol. 2015; 20:207–239. PMID: 25782566.

9. Hohenberger W, Weber K, Matzel K, Papadopoulos T, Merkel S. Standardized surgery for colonic cancer: complete mesocolic excision and central ligation--technical notes and outcome. Colorectal Dis. 2009; 11:354–364. PMID: 19016817.
10. Nascimbeni R, Burgart LJ, Nivatvongs S, Larson DR. Risk of lymph node metastasis in T1 carcinoma of the colon and rectum. Dis Colon Rectum. 2002; 45:200–206. PMID: 11852333.

11. Beaton C, Twine CP, Williams GL, Radcliffe AG. Systematic review and meta-analysis of histopathological factors influencing the risk of lymph node metastasis in early colorectal cancer. Colorectal Dis. 2013; 15:788–797. PMID: 23331927.

12. General rules for clinical and pathological studies on cancer of the colon, rectum and anus. Part I. Clinical classification. Japanese Research Society for Cancer of the Colon and Rectum. Jpn J Surg. 1983; 13:557–573. PMID: 6672390.
13. Ovrebo K, Rokke O. Extended lymph node dissection in colorectal cancer surgery. Reliability and reproducibility in assessments of operative reports. Int J Colorectal Dis. 2010; 25:213–222. PMID: 19865821.

14. Storli KE, Sondenaa K, Furnes B, Nesvik I, Gudlaugsson E, Bukholm I, et al. Short term results of complete (D3) vs. standard (D2) mesenteric excision in colon cancer shows improved outcome of complete mesenteric excision in patients with TNM stages I-II. Tech Coloproctol. 2014; 18:557–564. PMID: 24357446.

15. Galizia G, Lieto E, De Vita F, Ferraraccio F, Zamboli A, Mabilia A, et al. Is complete mesocolic excision with central vascular ligation safe and effective in the surgical treatment of right-sided colon cancers? A prospective study? Int J Colorectal Dis. 2014; 29:89–97. PMID: 23982425.

16. Bertelsen CA, Neuenschwander AU, Jansen JE, Wilhelmsen M, Kirkegaard-Klitbo A, Tenma JR, et al. Disease-free survival after complete mesocolic excision compared with conventional colon cancer surgery: a retrospective, population-based study. Lancet Oncol. 2015; 16:161–168. PMID: 25555421.

17. Willaert W, Ceelen W. Extent of surgery in cancer of the colon: is more better? World J Gastroenterol. 2015; 21:132–138. PMID: 25574086.

18. Zheng M, Ma J, Fingerhut A, Adamina MP, Atroschenko A, Bergamaschi R, et al. Complete mesocolic excision for colonic cancer: Society for Translational Medicine expert consensus statement. Ann Laparosc Endosc Surg. 2018; 3:68.

19. Lu JY, Xu L, Xue HD, Zhou WX, Xu T, Qiu HZ, et al. The Radical Extent of lymphadenectomy - D2 dissection versus complete mesocolic excision of LAparoscopic Right Colectomy for right-sided colon cancer (RELARC) trial: study protocol for a randomized controlled trial. Trials. 2016; 17:582. PMID: 27931247.

20. International Prospective Observational Cohort Study for Optimal Bowel Resection Extent and Central Radicality for Colon Cancer (T-REX). ClinicalTrials.gov identifier: NCT02938481 [Internet]. Bethesda (MD): ClinicalTrials.gov;cited 2019 Jul 9. Available from: https://clinicaltrials.gov/ct2/show/NCT02938481.
Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download