This article has been
cited by other articles in ScienceCentral.
Abstract
Purpose
Appendiceal tumoral lesions can occur as benign, malignant, or borderline disease. Determination of the extent of surgery through accurate diagnosis is important in these tumoral lesions. In this study, we assessed the accuracy of preoperative CT and identified the factors affecting diagnosis.
Methods
Patients diagnosed or strongly suspected from July 2016 to June 2019 with appendiceal mucocele or mucinous neoplasm using abdominal CT were included in the study. All the patients underwent single-incision laparoscopic cecectomy with the margin of cecum secured at least 2 cm from the appendiceal base. To compare blood test results and CT findings, the patients were divided into a mucinous and a nonmucinous group according to pathology.
Results
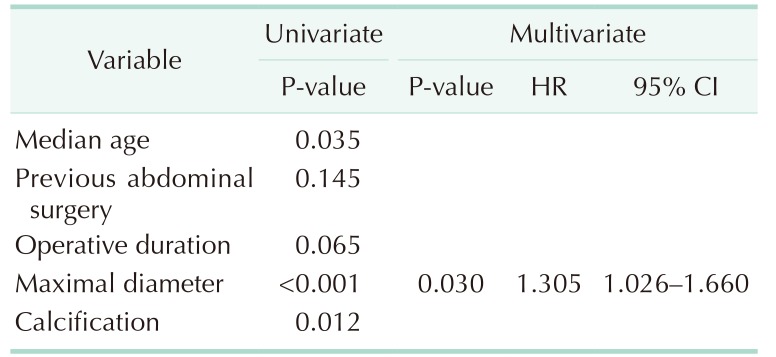
The total number of patients included in this study was 54 and biopsy confirmed appendiceal mucinous neoplasms in 39 of them. With CT, the accuracy of diagnosis was 89.7%. The mean age of the mucinous group was greater than that of the nonmucinous group (P = 0.035). CT showed that the maximum diameter of appendiceal tumor in the mucinous group was greater than that in the nonmucinous group (P < 0.001). Calcification was found only in the appendix of patients in the mucinous group (P = 0.012). Multivariate analysis revealed that lager tumor diameter was a factor of diagnosis for appendiceal mucinous neoplasm.
Conclusion
The accuracy of preoperative diagnosis of appendiceal mucinous neoplasms in this study was 89.7%. Blood test results did not provide differential diagnosis, and the larger the diameter of appendiceal tumor on CT, the more accurate the diagnosis.
Go to :

Keywords: Appendix, Diagnosis, Mucinous neoplasm
INTRODUCTION
The appendix is the organ most commonly involved in inflammatory abdominal diseases. Although the incidence of tumoral lesions is low, they can occur as benign, malignant, or borderline disease. In the surgical treatment of tumoral lesions, determination of the extent of surgery through accurate diagnosis is important.
Appendiceal mucocele, defined as abnormal mucin accumulation in the appendiceal lumen, was first described in 1842 by von Rokitansky [
1]. In 2016, a consensus for classification and pathologic reporting of pseudomyxoma peritonei (PMP) and associated appendiceal neoplasia [
2] was reached, and it enabled the uniform use of terminology. According to recent consensus, low-grade appendiceal mucinous neoplasm (LAMN) refers to low-grade cytologic atypia, pushing invasion through appendiceal wall with resultant breach of muscularis mucosae, and if cytologically high-grade atypia is observed, the lesion is termed a high-grade appendiceal mucinous neoplasm (HAMN) [
3]. Appendiceal mucocele is caused by neoplastic transformation of goblet cells, which leads to the formation of a mucinous tumor, and the amount of mucin increases as the tumor grows. A mucocele is formed following intraluminal accumulation of mucin [
4].
Generally, CT scan shows a low-attenuation, well-encapsulated mass with smooth regular walls in the right lower quadrant. The presence of curvilinear calcified walls in this area strongly supports the diagnosis. The calcification is a result of a chronic inflammatory process induced by the mucus in the appendiceal wall [
5]. About 25% of the lesions are asymptomatic, and they are incidental findings at medical check-ups and surgeries [
6]. The authors added immunological cells for diagnosis, which have not been included as a basic test for differential diagnosis in appendiceal mucinous neoplasm. This test is one of methods carried out recently to confirm the diagnosis and function of various immune or inflammatory diseases [
7].
This study compared and analyzed perioperative blood and imaging tests on appendiceal tumors that were incidental findings. The aim of the study was to identify factors that affect diagnosis and to confirm factors that may increase diagnostic rate.
Go to :

METHODS
Patients diagnosed or strongly suspected from July 2016 to June 2019 with appendiceal mucocele or mucinous neoplasm using abdominal CT were included. Total colonoscopy and assessment for tumor markers, such as CEA, were performed prior to surgery. All the operations were performed by a single surgeon, and all the patients underwent single-incision laparoscopic cecectomy with the margin of the cecum secured at least 2 cm from the appendiceal base. The cecum was resected using a 60-mm endoscopic linear stapler (gold cartilage, Echelon, Ethicon, Johnson & Johnson, New Brunswick, NJ, USA). The resected specimen was put into an endo-pouch in the abdominal cavity and taken out through a midline incision.
The patients were divided into a mucinous group and a nonmucinous group according to the postoperative pathologic diagnosis of mucinous neoplasm or nonmucinous neoplasm to compare blood test results (WBC counts, CRP levels, and CEA levels) and CT findings (maximum diameter of appendiceal lesion, presence or absence of calcification) between patient groups. Immunologic cells (γδ T cells, αβ T cells, T cells, and mucosal-associated invariant T cells) were analyzed prospectively in addition to the routine tests to increase the accuracy of diagnosis after patient consent. Patients with diseases that may directly affect immune cell populations, such as autoimmune diseases, uncontrolled diabetes mellitus, and tuberculosis, and patients with diseases that require treatments that affect immune cells, such as immunosuppressant therapy, were excluded from the study. Immune cell populations were measured based on the presence of cell surface antigens using a cell analyzer by separating mononuclear cells from peripheral blood and cancer tissue samples, staining them for 20 minutes with various monoclonal antibodies, and then analyzing by flow cytometry.
By conducting multivariate analysis of factors that alter the parameters, the researchers attempted to find a factor that may be indicative of a mucinous neoplasm.
All the patients were fully informed, and consent was obtained prior to enrollment. This study was approved by the Institutional Review Board of the Chonnam National University Hospital (CNUH-2018-076).
Statistical analysis
Differences between the 2 groups were compared using Student t-test or the Mann-Whitney U-test for continuous data or the chi-square test or Fisher exact test for categorical data. For multivariate analyses, we fit binary logistic regression models to identify significant risk factors from a set of significant main-effect variables (P < 0.15). Statistical analyses were performed using IBM SPSS Statistics ver. 20.0 (IBM Co., Armonk, NY, USA). P-values ≤0.05 were considered statistically significant.
Go to :

RESULTS
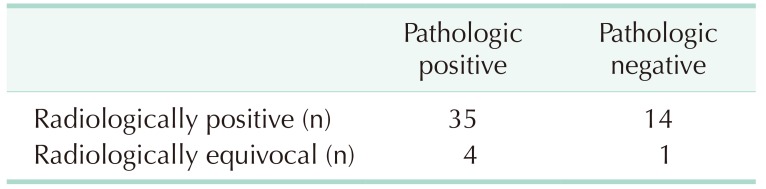
Mucinous neoplasm was diagnosed or strongly suspected in 54 patients using preoperative CT, and all the patients underwent surgical treatment. Biopsy results confirmed a diagnosis of appendiceal mucinous neoplasms in 39 patients. With CT, the accuracy of diagnosis was 89.7% (35 of 39), the sensitivity was 89.7% (35 of 39), and the specificity was 6.7% (1 of 15). The positive predictive value was 71.4% (35 of 49) and the negative predictive value was 20% (1 of 5) (
Table 1).
Table 1
Discrepancy between perioperative diagnosis

The mean age of the mucinous group was greater than that of the nonmucinous group (57.3 years vs. 65.3 years, P = 0.035). There were no statistically significant differences in other patient characteristics, such as sex, body mass index, American Society of Anesthesiologists physical status classification, medical history, and history of abdominal surgery (
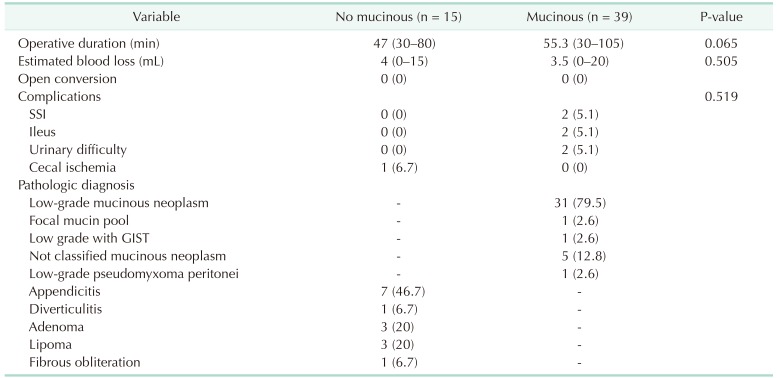
Table 2). The mean operating time from skin incision to skin closure was 54.9 minutes with no difference in operative duration, estimated blood loss, and short-term postoperative complications. The complications that occurred include surgical site infection, ileus, urinary difficulty, and cecal ischemia. Open conversion was not performed in any patient. The most common misdiagnosis was appendicitis (7 out of 15 cases, 46.7%). The other confirmed misdiagnoses were diverticulitis, adenoma, lipoma, and fibrous obliteration (
Table 3).
Table 2
Patients' characteristics
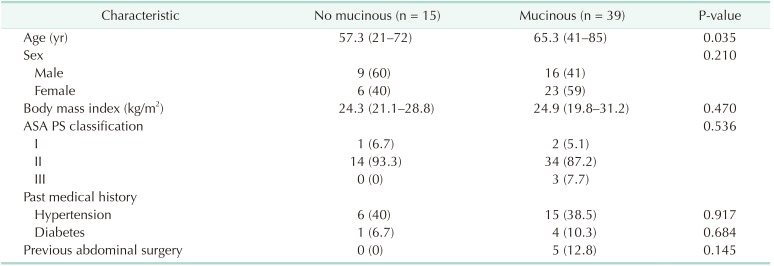

Table 3
Perioperative outcome

There were no statistically significant differences in the results of the preoperative blood tests (WBC counts, CRP levels, and immunologic cell counts). The mucinous group had higher CEA levels than the nonmucinous group, although there were no statistically significant differences between the groups (2.52 vs. 9.47, P = 0.359) (
Table 4). CT scan showed that the mucinous group had a higher maximum diameter of lesion than the nonmucinous group (9.33 mm vs. 26.38 mm, P < 0.001) (
Fig. 1). Calcification within the appendix was observed only in the mucinous group (P = 0.012) (
Table 4). Multivariate analysis showed that the larger the diameter of the lesion, the higher the accuracy of the diagnosis (hazard ratio, 1.305; 95% confidence interval, 1.026–1.660; P = 0.03) (
Table 5).
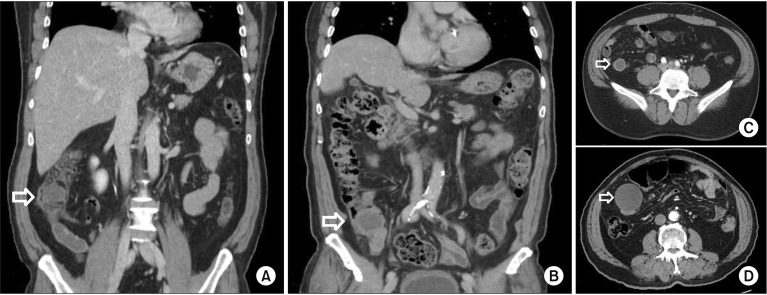 | Fig. 1(A) Base of lipoma, (B) base of mucinous neoplasm, (C) tip of lipoma, (D) tip of mucinous neoplasm. Nonmucinous tumor shows similar pattern with mucinous neoplasm at appendiceal base (A, C), but overall size of the tip was smaller (C, D).
|
Table 4
Perioperative blood test and CT findings
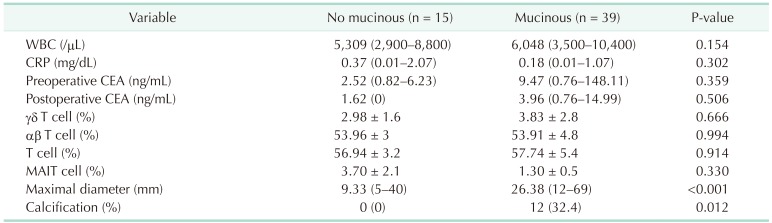

Table 5
Predictive factors of appendiceal mucinous neoplasm

Go to :

DISCUSSION
This study showed that patients with appendiceal mucinous neoplasms had a higher median age than patients with nonmucinous neoplasms, and differential diagnosis could not be made on the basis of the included blood tests. Large diameter of lesion and calcification on CT scan were indicative of appendiceal mucinous neoplasm. The diameter of the appendiceal tumor was a predictive factor.
Appendiceal mucinous neoplasm is not easy to diagnose through preoperative evaluation due to overlapping imaging appearances or through routine blood tests with other appendiceal diseases. Elevated CEA could be meaningful in mucinous neoplasm for recurrence [
8], but this study did not show any difference between the 2 groups for early detection. Although only less than 50% of the cases are identified, ultrasound shows characteristic curvilineal calcifications [
9]. A specific pattern in which echogenic layered internal content and acoustic shadowing from mural calcifications, also called “onion skin sign,” strongly suggests appendiceal mucinous neoplasm. When a separate normal ovary can be distinguished, it is diagnosed more accurate [
10]. CT shows a dilated appendix with low-attenuation cystic round or tubular lesion, which is also accompanied by a wall calcification of up to 50% [
511]. Some authors suggested it may identify appendiceal mucocele from acute appendicitis prospectively under conditions such as cystic dilation of the appendix, mural calcification, and maximum luminal diameter > 1.3 cm [
1213]. A study showed calcification or luminal diameter greater than 2 cm were prognostic factors for appendiceal neoplasm [
14]. The analysis results of 49 mucocele patients, which were recently published, also indicated that the underlying neoplasm is strongly suspected if it is accompanied by mural calcification, a diameter > 2 cm, and not periappendiceal stranding [
15]. At MRI, a mucocele shows various T1-weighted intensity depending on the specific protein content, but T2-weighted imaging has hyperintense for cystic mass [
3]. MRI is useful for ruptured appendiceal lesions because extraluminal or extravasated mucin shows hyperintense on T2-weighted imaging [
13].
The exact function of the appendix is still unclear, but it has always been considered to have an immunological function. It has also been recently acknowledged that the appendix, which is not exactly in the direction of the passing feces, may play the immunologic role of maintaining the reservoir of normal gut flora [
1617]. Appendiceal mucinous tumor is a controversial lesion. First, the classification system is constantly being revised. It can be classified as benign or malignant based on mucin secretion [
18]. Higa et al. [
19] evaluated 73 appendiceal lesions and identified 3 distinct clinicopathologic types of mucoceles, which are as follows: (1) hyperplastic polyp, which shows focal or diffuse mucosal hyperplasia with no epithelial atypia and mild distension of the lumen, (2) mucinous cystadenoma, which shows some degree of epithelial atypia and marked distension of the lumen, and (3) mucinous cystadenocarcinoma (PMP). However, in 2016, an international oncology group comprising 71 members representing 13 countries issued a consensus statement on the nomenclature and classification of appendiceal mucinous tumors, PMPs, and GCTs based on histologic type and biologic behavior with implications for staging and treatment. In this new guideline, appendiceal mucinous neoplasms were classified into 4, namely adenomas, LAMNs, HAMNs, and mucinous adenocarcinomas. The histologic classification of PMPs was different from that of primary appendiceal mucinous tumors. PMPs were classified into 4, namely acellular mucin, low-grade, high-grade, and high-grade with signet ring cells [
2].
There are no clear guidelines on the extent of surgical resection; therefore, many reports on surgical procedures have been published [
20]. Some reports claim that laparoscopic surgery is possible [
21], but others maintain that laparotomy is better than laparoscopic appendectomy if mucocele is suspected based on preoperative diagnostic imaging results [
22]. In a study with a larger sample size than the case report mentioned above, 135 patients were divided into groups of appendectomy, right hemicolectomy, and more extensive procedure, and the study report claimed that a lesion should be considered premalignant if its size is greater than 2 cm [
23]. In more recent reports, mucoceles were considered benign; therefore, open conversion to prevent dissemination of malignant cells was not necessary. In this study, cytological examination of intra-abdominal fluid and resection of enlarged lymph nodes were necessary, and right hemicolectomy was considered when malignant cells were found in the lymph nodes. The study also reported that cytoreductive surgery and intraperitoneal chemotherapy were necessary if epithelial cells were found in the fluid [
24]. More importantly, it was necessary for the pathologist to thoroughly check the lesion for perforation and follow-up with all the patients because of the possibility of recurrence, colorectal cancer, and PMP [
25].
This study was conducted prospectively as blood tests of immune cells may have diagnostic value, but the tests did not confirm any meaningful results. Previously published studies show differences between immunological or inflammatory diseases and healthy adults. However, it appears to have no difference because this study was designed to diagnose differentially among the diseased cases. Although the institution is a tertiary referral center, the study was limited by insufficient number of patients because the condition under investigation is rare. Until recently, appendiceal mucinous neoplasms were mostly reported in case series with pathological findings or in case reports that consider laparoscopic resection of the lesion possible. In this study, single-incision laparoscopic operations were performed in all the patients, and the resection included the cecum at a reasonable distance from the appendiceal base so as to secure a negative tumor margin. Endo-pouch was also used to prevent spillage. Justification for the surgical method and extent of surgery will be made in the near future by analysis of long-term outcomes. In addition, sufficient cases should be collected to provide a clear specific size in CT through subsequent or multicenter studies.
In conclusion, even though high-resolution CT has recently been used for diagnosis, accurate preoperative diagnosis of appendiceal mucinous neoplasms remains challenging. This study confirmed that the greater the diameter of the lesion as observed on CT scan, the more accurate the diagnosis.
Go to :

ACKNOWLEDGEMENTS
This study was supported by a grant from the Biomedical Research Institute of Chonnam National University Hospital (CRI 18012-1).
Go to :

Notes
Go to :

References
1. von Rokitansky C. A manual of pathological anatomy. Philadelphia (PA): Blanchard & Lea;1855.
2. Carr NJ, Cecil TD, Mohamed F, Sobin LH, Sugarbaker PH, Gonzalez-Moreno S, et al. A consensus for classification and pathologic reporting of pseudomyxoma peritonei and associated appendiceal neoplasia: the results of the Peritoneal Surface Oncology Group International (PSOGI) Modified Delphi Process. Am J Surg Pathol. 2016; 40:14–26. PMID:
26492181.
3. Leonards LM, Pahwa A, Patel MK, Petersen J, Nguyen MJ, Jude CM. Neoplasms of the appendix: pictorial review with clinical and pathologic correlation. Radiographics. 2017; 37:1059–1083. PMID:
28598731.

4. Hinson FL, Ambrose NS. Pseudomyxoma peritonei. Br J Surg. 1998; 85:1332–1339. PMID:
9782010.

5. Madwed D, Mindelzun R, Jeffrey RB Jr. Mucocele of the appendix: imaging findings. AJR Am J Roentgenol. 1992; 159:69–72. PMID:
1609724.

6. Isaacs KL, Warshauer DM. Mucocele of the appendix: computed tomographic, endoscopic, and pathologic correlation. Am J Gastroenterol. 1992; 87:787–789. PMID:
1590322.
7. Won EJ, Ju JK, Cho YN, Jin HM, Park KJ, Kim TJ, et al. Clinical relevance of circulating mucosal-associated invariant T cell levels and their anti-cancer activity in patients with mucosal-associated cancer. Oncotarget. 2016; 7:76274–76290. PMID:
27517754.

8. Landen S, Bertrand C, Maddern GJ, Herman D, Pourbaix A, de Neve A, et al. Appendiceal mucoceles and pseudomyxoma peritonei. Surg Gynecol Obstet. 1992; 175:401–404. PMID:
1440166.
9. Pickhardt PJ, Levy AD, Rohrmann CA Jr, Kende AI. Primary neoplasms of the appendix: radiologic spectrum of disease with pathologic correlation. Radiographics. 2003; 23:645–662. PMID:
12740466.

10. Caspi B, Cassif E, Auslender R, Herman A, Hagay Z, Appelman Z. The onion skin sign: a specific sonographic marker of appendiceal mucocele. J Ultrasound Med. 2004; 23:117–121. PMID:
14756359.
11. Dachman AH, Lichtenstein JE, Friedman AC. Mucocele of the appendix and pseudomyxoma peritonei. AJR Am J Roentgenol. 1985; 144:923–929. PMID:
3885692.

12. Bennett GL, Tanpitukpongse TP, Macari M, Cho KC, Babb JS. CT diagnosis of mucocele of the appendix in patients with acute appendicitis. AJR Am J Roentgenol. 2009; 192:W103–W110. PMID:
19234237.

13. Van Hooser A, Williams TR, Myers DT. Mucinous appendiceal neoplasms: pathologic classification, clinical implications, imaging spectrum and mimics. Abdom Radiol (NY). 2018; 43:2913–2922. PMID:
29564494.

14. Carr NJ, McCarthy WF, Sobin LH. Epithelial noncarcinoid tumors and tumor-like lesions of the appendix. A clinicopathologic study of 184 patients with a multivariate analysis of prognostic factors. Cancer. 1995; 75:757–768. PMID:
7828125.

15. Marotta B, Chaudhry S, McNaught A, Quereshy F, Vajpeyi R, Chetty R, et al. Predicting underlying neoplasms in appendiceal mucoceles at CT: focal versus diffuse luminal dilatation. AJR Am J Roentgenol. 2019; 213:343–348. PMID:
30973782.

16. Randal Bollinger R, Barbas AS, Bush EL, Lin SS, Parker W. Biofilms in the large bowel suggest an apparent function of the human vermiform appendix. J Theor Biol. 2007; 249:826–831. PMID:
17936308.

17. Laurin M, Everett ML, Parker W. The cecal appendix: one more immune component with a function disturbed by post-industrial culture. Anat Rec (Hoboken). 2011; 294:567–579. PMID:
21370495.

18. Woodruff R. Benign and malignant cyst tumors of the appendix. Surg Gynecol Obstet. 1940; 71:750–755.
19. Higa E, Rosai J, Pizzimbono CA, Wise L. Mucosal hyperplasia, mucinous cystadenoma, and mucinous cystadenocarcinoma of the appendix. A re-evaluation of appendiceal “mucocele”. Cancer. 1973; 32:1525–1541. PMID:
4757938.
20. De Abreu Filho JG, De Lira EF. Mucocele of the appendix: appendectomy or colectomy? J Coloproctol (Rio J). 2011; 31:276–284.
21. Miraliakbari R, Chapman WH 3rd. Laparoscopic treatment of an appendiceal mucocele. J Laparoendosc Adv Surg Tech A. 1999; 9:159–163. PMID:
10235354.

22. Gonzalez Moreno S, Shmookler BM, Sugarbaker PH. Appendiceal mucocele. Contraindication to laparoscopic appendectomy. Surg Endosc. 1998; 12:1177–1179. PMID:
9716778.
23. Stocchi L, Wolff BG, Larson DR, Harrington JR. Surgical treatment of appendiceal mucocele. Arch Surg. 2003; 138:585–589. PMID:
12799327.

24. Dhage-Ivatury S, Sugarbaker PH. Update on the surgical approach to mucocele of the appendix. J Am Coll Surg. 2006; 202:680–684. PMID:
16571440.

25. Ruiz-Tovar J, Teruel DG, Castineiras VM, Dehesa AS, Quindos PL, Molina EM. Mucocele of the appendix. World J Surg. 2007; 31:542–548. PMID:
17318706.

Go to :






 PDF
PDF ePub
ePub Citation
Citation Print
Print








 XML Download
XML Download