Abstract
Hepatic hydrothorax is a transudative pleural effusion that complicates advanced liver cirrhosis. Patients refractory to medical treatment plus salt restriction and diuretics are considered to have refractory hepatic hydrothorax and may require transjugular intrahepatic portosystemic shunt (TIPS) or liver transplant. Successful antiviral therapy reduces the incidence of some complications of cirrhosis secondary to HCV infection. We report a case of hepatic hydrothorax in a 55-year-old female patient with HCV cirrhosis, which exhibited a spontaneous decrease in pleural effusion after direct antiviral agent (DAA) therapy. In cases of HCV cirrhosis, DAAs are worth administering before treatment by TIPS or liver transplantation.
References
1. Badillo R, Rockey DC. Hepatic hydrothorax: clinical features, management, and outcomes in 77 patients and review of the literature. Medicine (Baltimore). 2014; 93:135–142.
3. Roussos A, Philippou N, Mantzaris GJ, Gourgouliannis KI. Hepatic hydrothorax: pathophysiology diagnosis and management. J Gastroenterol Hepatol. 2007; 22:1388–1393.

4. Al-Zoubi RK, Abu Ghanimeh M, Gohar A, Salzman GA, Yousef O. Hepatic hydrothorax: clinical review and update on consensus guidelines. Hosp Pract (1995). 2016; 44:213–223.

5. Huang PM, Chang YL, Yang CY, Lee YC. The morphology of diaphragmatic defects in hepatic hydrothorax: thoracoscopic finding. J Thorac Cardiovasc Surg. 2005; 130:141–145.

6. Ditah IC, Al Bawardy BF, Saberi B, Ditah C, Kamath PS. Transjugular intrahepatic portosystemic stent shunt for medically refractory hepatic hydrothorax: a systematic review and cumulative metaanalysis. World J Hepatol. 2015; 7:1797–1806.

7. Riggio O, Merlli M, Pedretti G, et al. Hepatic encephalopathy after transjugular intrahepatic portosystemic shunt. Incidence and risk factors. Dig Dis Sci. 1996; 41:578–584.
8. Hou F, Qi X, Guo X. Effectiveness and safety of pleurodesis for hepatic hydrothorax: a systematic review and metaanalysis. Dig Dis Sci. 2016; 61:3321–3334.

9. Jin YJ, Lee JW, Lee JI, et al. Efficacy and tolerability of peginterferon alpha plus ribavirin in the routine daily treatment of chronic hepatitis C patients in Korea: a multicenter, retrospective observational study. Gut Liver. 2012; 6:98–106.

10. Jin YJ, Lee JW, Lee JI, et al. Multicenter comparison of PEG-IFN α2a or α2b plus ribavirin for treatmentnaïve HCV patient in Korean population. BMC Gastroenterol. 2013; 13:74.

11. Dusheiko G. The impact of antiviral therapy for hepatitis C on the quality of life: a perspective. Liver Int. 2017; 37(Suppl 1):7–12.

12. HCV guidance: recommendations for testing, managing, and treating hepatitis C. [Internet]. Alexandria (VA): American Association for the Study of Liver Diseases;2017 Sep 21. [cited 2019 Jul 25]. Available from:. https://www.hcvguidelines.org/unique-populations/decompensated-cirrhosis.
13. El-Sherif O, Jiang ZG, Tapper EB, et al. Baseline factors associated with improvements in decompensated cirrhosis after direct-acting antiviral therapy for hepatitis C virus infection. Gastroenterology. 2018; 154:2111–2121.e8.
15. Wyles DL, Ruane PJ, Sulkowski MS, et al. Daclatasvir plus sofosbuvir for HCV in patients coinfected with HIV-1. N Engl J Med. 2015; 373:714–725.
16. Sulkowski MS, Naggie S, Lalezari J, et al. Sofosbuvir and ribavirin for hepatitis C in patients with HIV coinfection. JAMA. 2014; 312:353–361.

17. Singh S, Facciorusso A, Loomba R, Falck-Ytter YT. Magnitude and kinetics of decrease in liver stiffness after antiviral therapy in patients with chronic hepatitis C: a systematic review and metaanalysis. Clin Gastroenterol Hepatol. 2018; 16:27–38.e4.
18. Afdhal N, Zeuzem S, Kwo P, et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014; 370:1889–1898.

19. Libânio D, Marinho RT. Impact of hepatitis C oral therapy in portal hypertension. World J Gastroenterol. 2017; 23:4669–4674.

Fig. 1.
Abdomen computed tomography finding at admission. The liver was shrunken and showed surface nodularity, and a moderate amount of ascites was noted near the liver. Right massive pleural effusion post pigtail catheter drainage.
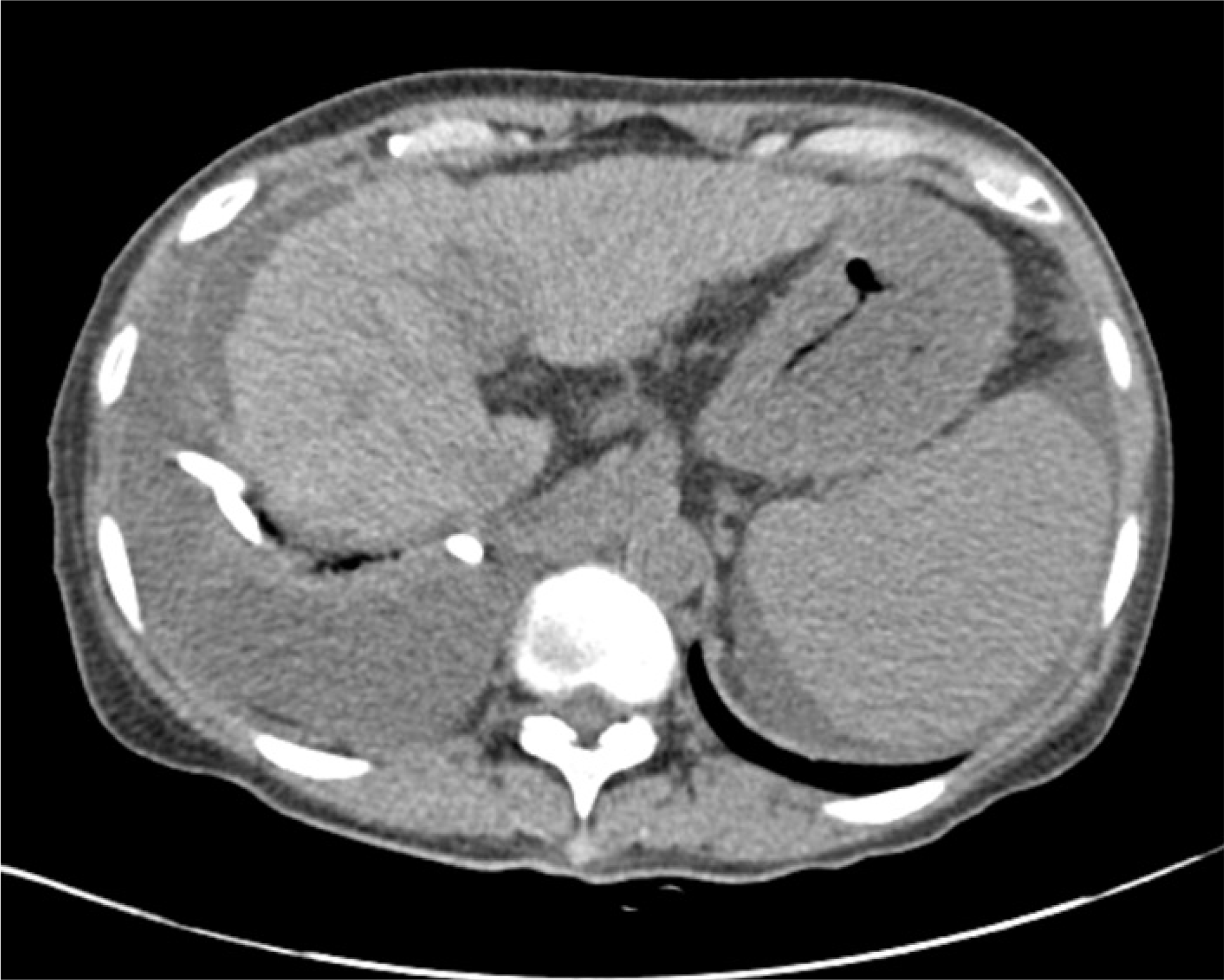
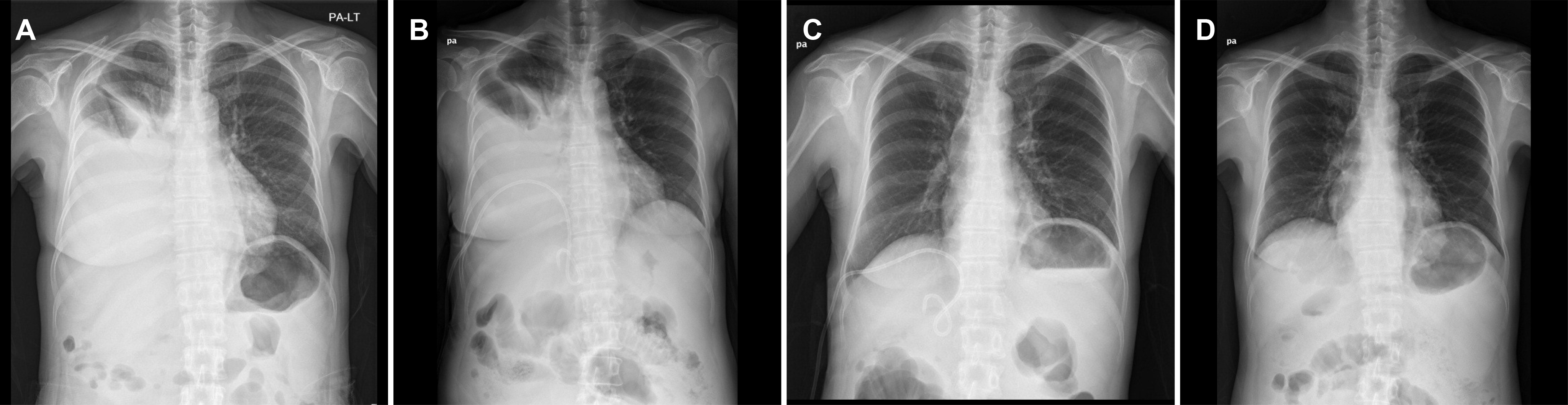
Fig. 2.
Serial chest X-ray findings of hepatic hydrothorax. (A) A large amount of fluid had collected in the right pleural cavity at admission. (B) Insertion of a pigtail catheter in the right pleural cavity (C) resulted in complete effusion drainage. (D) At 1 month after discharge, no residual effusion was noted. PA, posteroanterior.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download