Abstract
Optimal bowel preparation is essential for a more accurate, comfortable, and safe colonoscopy. The majority of postcolonoscopy colorectal cancers can be explained by procedural factors, mainly missed polyps or inadequate examination. Therefore the most important goal of optimal bowel preparation is to reduce the incidence of colorectal cancer. Although adequate preparation should be achieved in 85–90% or more of all colonoscopy as a quality indicator, unfortunately 20–30% shows inadequate preparation. Laxatives for oral colonoscopy bowel preparation can be classified into polyethylene glycol (PEG)-electrolyte lavage solution, osmotic laxatives, stimulant laxatives, and divided into high-volume solution (≥3 L) and low-volume solution (<3 L). The updated 2019 European Society of Gastrointestinal Endoscopy (ESGE) guideline is broadly similar to the 2014 American Society for Gastrointestinal Endoscopy (ASGE) recommendations and reaffirms the importance of split-dosing. However, new ESGE guideline, unlike the 2014 ASGE recommendation, suggests the use of high volume or low volume PEG-based regimens as well as that of non-PEG based agents that have been clinically validated for most outpatient scenarios. For effective, safe, and highly adherent bowel preparation, physicians who prescribe and implement colonoscopy should properly know the advantages and limitations, the dosing, and the timing of regimens. Recently many studies have attempted to find the most ideal regimens, and more convenient, effective, and safe regimens have been developed by reducing the dosing volume and improving the taste. The high tolerability and acceptability of the new low-volume regimens suggest us how we should use it to increase the participation of the national colorectal cancer screening program.
Go to : 
References
1. Johnson DA, Barkun AN, Cohen LB, et al. Optimizing adequacy of bowel cleansing for colonoscopy: recommendations from the U.S. multisociety task force on colorectal cancer. Gastrointest Endosc. 2014; 80:543–562.

2. Froehlich F, Wietlisbach V, Gonvers JJ, Burnand B, Vader JP. Impact of colonic cleansing on quality and diagnostic yield of colonoscopy: the European Panel of Appropriateness of Gastrointestinal Endoscopy European multicenter study. Gastrointest Endosc. 2005; 61:378–384.

3. Clark BT, Protiva P, Nagar A, et al. Quantification of adequate bowel preparation for screening or surveillance colonoscopy in men. Gastroenterology. 2016; 150:396–405.

4. Hong SN, Sung IK, Kim JH, et al. The effect of the bowel preparation status on the risk of missing polyp and adenoma during screening colonoscopy: a tandem colonoscopic study. Clin Endosc. 2012; 45:404–411.

5. le Clercq CM, Bouwens MW, Rondagh EJ, et al. Postcolonoscopy colorectal cancers are preventable: a population-based study. Gut. 2014; 63:957–963.

6. Kaminski MF, Thomas-Gibson S, Bugajski M, et al. Performance measures for lower gastrointestinal endoscopy: a European Society of Gastrointestinal Endoscopy (ESGE) quality improvement initiative. Endoscopy. 2017; 49:378–397.

7. Harewood GC, Sharma VK, de Garmo P. Impact of colonoscopy preparation quality on detection of suspected colonic neoplasia. Gastrointest Endosc. 2003; 58:76–79.

8. Hassan C, East J, Radaelli F, et al. Bowel preparation for colonoscopy: European Society of Gastrointestinal Endoscopy (ESGE) guideline – update 2019. Endoscopy. 2019; 51:775–794.

9. McLachlan SA, Clements A, Austoker J. Patients' experiences and reported barriers to colonoscopy in the screening context–a systematic review of the literature. Patient Educ Couns. 2012; 86:137–146.

10. DiPalma JA, Brady CE 3rd. Colon cleansing for diagnostic and surgical procedures: polyethylene glycol-electrolyte lavage solution. Am J Gastroenterol. 1989; 84:1008–1016.
11. Spadaccini M, Frazzoni L, Vanella G, et al. Efficacy and tolerability of high- vs low-volume split-dose bowel cleansing regimens for colonoscopy: a systematic review and metaanalysis. Clin Gastroenterol Hepatol. 2019 Nov 1. [Epub ahead of print].

12. Zawaly K, Rumbolt C, Abou-Setta AM, et al. The efficacy of split-dose bowel preparations for polyp detection: a systematic review and metaanalysis. Am J Gastroenterol. 2019; 114:884–892.

13. Davis GR, Santa Ana CA, Morawski SG, Fordtran JS. Development of a lavage solution associated with minimal water and electrolyte absorption or secretion. Gastroenterology. 1980; 78(5 Pt 1):991–995.

14. Marshall JB, Pineda JJ, Barthel JS, King PD. Prospective, randomized trial comparing sodium phosphate solution with polyethylene glycol-electrolyte lavage for colonoscopy preparation. Gastrointest Endosc. 1993; 39:631–634.

15. DiPalma JA, Marshall JB. Comparison of a new sulfate-free polyethylene glycol electrolyte lavage solution versus a standard solution for colonoscopy cleansing. Gastrointest Endosc. 1990; 36:285–289.

16. Rees DC, Kelsey H, Richards JD. Acute haemolysis induced by high dose ascorbic acid in glucose-6-phosphate dehydrogenase deficiency. BMJ. 1993; 306:841–842.

17. Xie Q, Chen L, Zhao F, et al. A metaanalysis of randomized controlled trials of low-volume polyethylene glycol plus ascorbic acid versus standard-volume polyethylene glycol solution as bowel preparations for colonoscopy. PLoS One. 2014; 9:e99092.

18. Moon CM, Park DI, Choe YG, et al. Randomized trial of 2-L polyethylene glycol + ascorbic acid versus 4-L polyethylene glycol as bowel cleansing for colonoscopy in an optimal setting. J Gastroenterol Hepatol. 2014; 29:1223–1228.
19. DeMicco MP, Clayton LB, Pilot J, Epstein MS. NOCT Study Group. Novel 1 L polyethylene glycol-based bowel preparation NER1006 for overall and right-sided colon cleansing: a randomized controlled phase 3 trial versus trisulfate. Gastrointest Endosc. 2018; 87:677–687.e3.
20. Bisschops R, Manning J, Clayton LB, Ng Kwet Shing R, Álvarez-González MM. MORA Study Group. Colon cleansing efficacy and safety with 1 L NER1006 versus 2 L polyethylene glycol + ascorbate: a randomized phase 3 trial. Endoscopy. 2019; 51:60–72.

21. Schreiber S, Baumgart DC, Drenth JPH, et al. Colon cleansing efficacy and safety with 1 L NER1006 versus sodium picosulfate with magnesium citrate: a randomized phase 3 trial. Endoscopy. 2019; 51:73–84.

22. Rex DK. Hyperosmotic low-volume bowel preparations: is NER1006 safe? Gastrointest Endosc. 2019; 89:656–658.

24. Bowel cleansing composition. [Internet]. Seoul: TAEJOON PHARM;2019 May 22. [cited 2020 Jan 21]. Available from:. http://kpat.kipris.or.kr/kpat/biblioa.do?method=biblioFrame.
25. Rex DK, Di Palma JA, Rodriguez R, McGowan J, Cleveland M. A randomized clinical study comparing reduced-volume oral sulfate solution with standard 4-liter sulfate-free electrolyte lavage solution as preparation for colonoscopy. Gastrointest Endosc. 2010; 72:328–336.

26. Yang HJ, Park SK, Kim JH, et al. Randomized trial comparing oral sulfate solution with 4-L polyethylene glycol administered in a split dose as preparation for colonoscopy. J Gastroenterol Hepatol. 2017; 32:12–18.

27. Kwak MS, Cha JM, Yang HJ, et al. Safety and efficacy of low-vol-ume preparation in the elderly: oral sulfate solution on the day before and split-dose regimens (SEE SAFE) study. Gut Liver. 2019; 13:176–182.

28. Ali IA, Roton D, Madhoun MF. Mo1077 oral sodium sulfate versus low volume peg for bowel preparation: a metaanalysis of randomized controlled trials. Gastrointest Endosc. 2019; 89:AB438.

29. Rex DK, DiPalma JA, McGowan J, Cleveland MV. A comparison of oral sulfate solution with sodium picosulfate: magnesium citrate in split doses as bowel preparation for colonoscopy. Gastrointest Endosc. 2014; 80:1113–1123.
30. Kim J, Kim HG, Kim KO, et al. Clinical comparison of low-volume agents (oral sulfate solution and sodium picosulfate with magnesium citrate) for bowel preparation: the EASE study. Intest Res. 2019; 17:413–418.

31. Yang HJ, Park DI, Park SK, et al. Novel sulfate tablet PBK-1701TC versus oral sulfate solution for colon cleansing: a randomized phase 3 trial. J Gastroenterol Hepatol. 2020; 35:29–36.
32. Vanner SJ, MacDonald PH, Paterson WG, Prentice RS, Da Costa LR, Beck IT. A randomized prospective trial comparing oral sodium phosphate with standard polyethylene glycol-based lavage solution (Golytely) in the preparation of patients for colonoscopy. Am J Gastroenterol. 1990; 85:422–427.
33. Cheng J, Tao K, Shuai X, Gao J. Sodium phosphate versus polyethylene glycol for colonoscopy bowel preparation: an updated metaanalysis of randomized controlled trials. Surg Endosc. 2016; 30:4033–4041.

34. Kastenberg D, Chasen R, Choudhary C, et al. Efficacy and safety of sodium phosphate tablets compared with PEG solution in colon cleansing: two identically designed, randomized, controlled, parallel group, multicenter phase III trials. Gastrointest Endosc. 2001; 54:705–713.

35. Markowitz GS, Stokes MB, Radhakrishnan J, D'Agati VD. Acute phosphate nephropathy following oral sodium phosphate bowel purgative: an underrecognized cause of chronic renal failure. J Am Soc Nephrol. 2005; 16:3389–3396.

36. Brunelli SM, Lewis JD, Gupta M, Latif SM, Weiner MG, Feldman HI. Risk of kidney injury following oral phosphosoda bowel preparations. J Am Soc Nephrol. 2007; 18:3199–3205.

37. Watts DA, Lessells AM, Penman ID, Ghosh S. Endoscopic and histologic features of sodium phosphate bowel preparation-induced colonic ulceration: case report and review. Gastrointest Endosc. 2002; 55:584–587.

38. van Lieshout I, Munsterman ID, Eskes AM, Maaskant JM, van der Hulst R. Systematic review and metaanalysis: sodium picosulphate with magnesium citrate as bowel preparation for colonoscopy. United European Gastroenterol J. 2017; 5:917–943.
39. Rocha RSP, Ribeiro IB, de Moura DTH, et al. Sodium picosulphate or polyethylene glycol before elective colonoscopy in outpatients? A systematic review and metaanalysis. World J Gastrointest Endosc. 2018; 10:422–441.

40. Jeon SR, Kim HG, Lee JS, et al. Randomized controlled trial of low-volume bowel preparation agents for colonic bowel preparation:2-L polyethylene glycol with ascorbic acid versus sodium picosulfate with magnesium citrate. Int J Colorectal Dis. 2015; 30:251–258.
41. Choi HS, Chung JW, Lee JW, et al. Polyethylene glycol plus ascorbic acid is as effective as sodium picosulfate with magnesium citrate for bowel preparation: a randomized trial. J Dig Dis. 2016; 17:268–273.

42. Seo SI, Kang JG, Kim HS, Jang MK, Kim HY, Shin WG. Efficacy and tolerability of 2-L polyethylene glycol with ascorbic acid versus sodium picosulfate with magnesium citrate: a randomized controlled trial. Int J Colorectal Dis. 2018; 33:541–548.

43. Hoy SM, Scott LJ, Wagstaff AJ. Sodium picosulfate/magnesium citrate: a review of its use as a colorectal cleanser. Drugs. 2009; 69:123–136.
44. Flemming JA, Vanner SJ, Hookey LC. Split-dose picosulfate, magnesium oxide, and citric acid solution markedly enhances colon cleansing before colonoscopy: a randomized, controlled trial. Gastrointest Endosc. 2012; 75:537–544.

45. Ze EY, Choi CH, Kim JW. Acute gastric injury caused by undis-solved sodium picosulfate/magnesium citrate powder. Clin Endosc. 2017; 50:87–90.

46. Spada C, Cesaro P, Bazzoli F, et al. Evaluation of Clensia®, a new low-volume PEG bowel preparation in colonoscopy: multicentre randomized controlled trial versus 4L PEG. Dig Liver Dis. 2017; 49:651–656.
47. Kump P, Hassan C, Spada C, et al. Efficacy and safety of a new low-volume PEG with citrate and simethicone bowel preparation for colonoscopy (Clensia): a multicenter randomized ob-server-blind clinical trial vs. a low-volume PEG with ascorbic acid (PEG-ASC). Endosc Int Open. 2018; 6:E907–E913.

48. Clark RE, Godfrey JD, Choudhary A, Ashraf I, Matteson ML, Bechtold ML. Low-volume polyethylene glycol and bisacodyl for bowel preparation prior to colonoscopy: a metaanalysis. Ann Gastroenterol. 2013; 26:319–324.

49. Tae CH, Jung SA, Na SK, et al. The use of low-volume polyethylene glycol containing ascorbic acid versus 2 L of polyethylene glycol plus bisacodyl as bowel preparation for colonoscopy. Scand J Gastroenterol. 2015; 50:1039–1044.

50. Baudet JS, Castro V, Redondo I. Recurrent ischemic colitis induced by colonoscopy bowel lavage. Am J Gastroenterol. 2010; 105:700–701.

51. Moolla M, Dang JT, Shaw A, et al. Simethicone decreases bloating and improves bowel preparation effectiveness: a systematic review and metaanalysis. Surg Endosc. 2019; 33:3899–3909.

52. Bucci C, Rotondano G, Hassan C, et al. Optimal bowel cleansing for colonoscopy: split the dose! A series of meta-analyses of controlled studies. Gastrointest Endosc. 2014; 80:566–576.e2.

53. Parsa N, Cockerell CJ, Matteson-Kome ML, et al. Mo1681 split-dose vs same-day bowel preparation for afternoon colonoscopies: a metaanalysis. Gastrointest Endosc. 2019; 89:AB519.

Go to : 
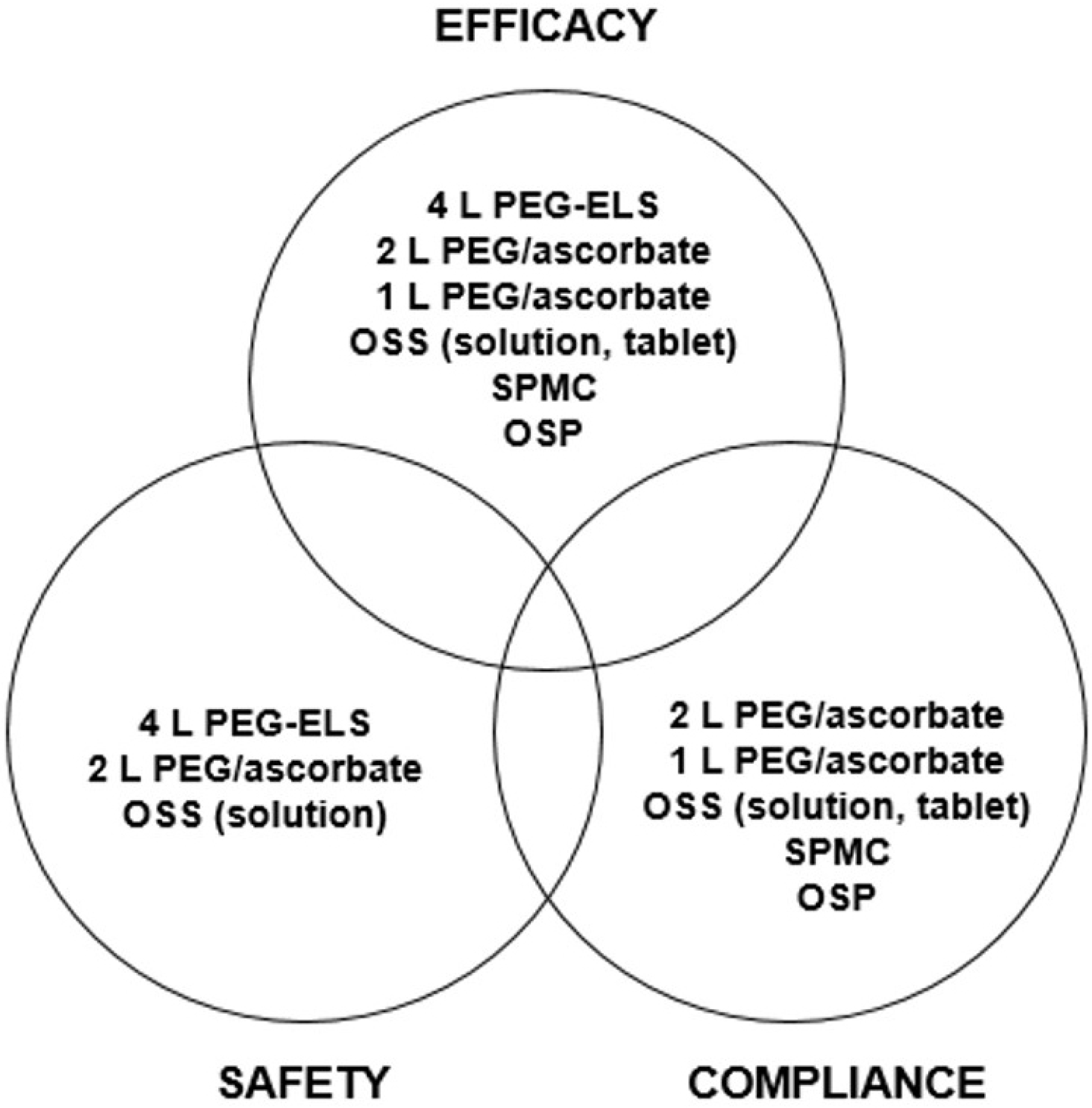 | Fig. 1.Factors for ideal bowel preparation and optimal choice of validated laxatives. PEG-ELS, polyethylene glycol-electrolyte lavage solution; OSS, oral sodium sulfate; SPMC, sodium picosulfate/ magnesium citrate; OSP, oral sodium phosphate. |
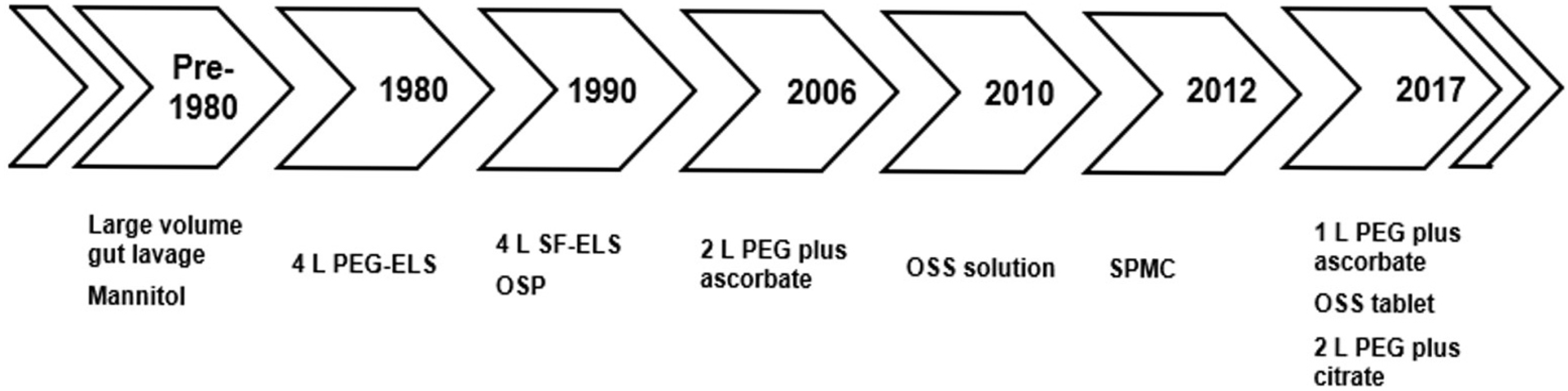 | Fig. 2.Developmental flow of the laxatives from old to new. PEG-ELS, polyethylene glycol-electrolyte lavage solution; SF-PEG, sulfate free-electrolyte lavage solution; OSP, oral sodium phosphate; OSS, oral sodium sulfate; SPMC, sodium picosulfate/magnesium citrate. |
Table 1.
Comparison of MFDS-approved Laxatives
MFDS, Ministry of Food and Drug Safety; PEG-ELS, polyethylene glycol-electrolyte lavage solution; OSS, oral sodium sulfate; OSP, oral sodium phosphate; SPMC, sodium picosulfate/magnesium citrate; Ccr, creatinine clearace; NYHA, New York Heat Association; CHF, congestive heart failure; IBD, inflammatory bowel disease; 6PDH, glucose-6-phosphate dehydrogenase.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download