1. Palacios C, Gonzalez L. Is vitamin D deficiency a major global public health problem? J Steroid Biochem Mol Biol. 2014; 144 Pt A:138–145. PMID:
24239505.

2. Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr. 2008; 87(4):1080S–1086S. PMID:
18400738.

3. Baggerly CA, Cuomo RE, French CB, Garland CF, Gorham ED, Grant WB, et al. Sunlight and vitamin D: necessary for public health. J Am Coll Nutr. 2015; 34(4):359–365. PMID:
26098394.

4. Pludowski P, Holick MF, Grant WB, Konstantynowicz J, Mascarenhas MR, Haq A, et al. Vitamin D supplementation guidelines. J Steroid Biochem Mol Biol. 2018; 175:125–135. PMID:
28216084.

5. Ashwell M, Stone EM, Stolte H, Cashman KD, Macdonald H, Lanham-New S, et al. UK food standards agency workshop report: an investigation of the relative contributions of diet and sunlight to vitamin D status. Br J Nutr. 2010; 104(4):603–611. PMID:
20522274.

6. Pearce SH, Cheetham TD. Diagnosis and management of vitamin D deficiency. BMJ. 2010; 340:b5664. PMID:
20064851.

7. Working Group of the Australian and New Zealand Bone and Mineral Society. Endocrine Society of Australia. Osteoporosis Australia. Vitamin D and adult bone health in Australia and New Zealand: a position statement. Med J Aust. 2005; 182(6):281–285. PMID:
15777143.
8. Khan SR, Whiteman DC, Kimlin MG, Janda M, Clarke MW, Lucas RM, et al. Effect of solar ultraviolet radiation exposure on serum 25(OH)D concentration: a pilot randomised controlled trial. Photochem Photobiol Sci. 2018; 17(5):570–577. PMID:
29619453.

9. Lee SH, Park SJ, Kim KM, Lee DJ, Kim WJ, Park RW, et al. Effect of sunlight exposure on serum 25-hydroxyvitamin D concentration in women with vitamin D deficiency: using ambulatory lux meter and sunlight exposure questionnaire. Korean J Fam Med. 2012; 33(6):381–389. PMID:
23267424.

10. Lovell GA, Byth JL, Craswell PW, Phillips PA, Thomas MJ. The influence of sunlight or dietary vitamin D on plasma 25‐hydroxyvitamin D in institutionalized elderly patients in a sub‐tropical climate. J Hum Nutr Diet. 1988; 1(3):163–170.

11. Wicherts IS, Boeke AJ, van der Meer IM, van Schoor NM, Knol DL, Lips P. Sunlight exposure or vitamin D supplementation for vitamin D-deficient non-western immigrants: a randomized clinical trial. Osteoporos Int. 2011; 22(3):873–882. PMID:
20683712.

13. Holick MF. Vitamin D deficiency. N Engl J Med. 2007; 357(3):266–281. PMID:
17634462.

14. Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011; 96(1):53–58. PMID:
21118827.

15. Youn JI, Choe YB, Park SB, Suh DH, Park YK, Ahn SK, et al. The Fitzpatrick skin type in Korean people. Korean J Dermatol. 2000; 38(7):920–927.
16. Webb AR, Kazantzidis A, Kift RC, Farrar MD, Wilkinson J, Rhodes LE. Colour counts: sunlight and skin type as drivers of vitamin D deficiency at UK latitudes. Nutrients. 2018; 10(4):E457. PMID:
29642423.

17. MacLaughlin J, Holick MF. Aging decreases the capacity of human skin to produce vitamin D3. J Clin Invest. 1985; 76(4):1536–1538. PMID:
2997282.

18. Holick MF. McCollum award lecture, 1994: vitamin D--new horizons for the 21st century. Am J Clin Nutr. 1994; 60(4):619–630. PMID:
8092101.

19. Brouwer-Brolsma EM, Vaes AM, van der Zwaluw NL, van Wijngaarden JP, Swart KM, Ham AC, et al. Relative importance of summer sun exposure, vitamin D intake, and genes to vitamin D status in Dutch older adults: the B-PROOF study. J Steroid Biochem Mol Biol. 2016; 164:168–176. PMID:
26275945.

20. Holick MF, MacLaughlin JA, Clark MB, Holick SA, Potts JT. Anderson RR, et al. Photosynthesis of previtamin D3 in human skin and the physiologic consequences. Science. 1980; 210:203–205. PMID:
6251551.
21. Bikle DD. Vitamin D metabolism, mechanism of action, and clinical applications. Chem Biol. 2014; 21(3):319–329. PMID:
24529992.

22. Anzenbacher P, Anzenbacherová E. Cytochromes P450 and metabolism of xenobiotics. Cell Mol Life Sci. 2001; 58(5-6):737–747. PMID:
11437235.

23. Morales E, Gascon M, Martinez D, Casas M, Ballester F, Rodríguez-Bernal CL, et al. Associations between blood persistent organic pollutants and 25-hydroxyvitamin D3 in pregnancy. Environ Int. 2013; 57-58:34–41. PMID:
23651836.

24. Yang JH, Lee YM, Bae SG, Jacobs DR Jr, Lee DH. Associations between organochlorine pesticides and vitamin D deficiency in the U.S. population. PLoS One. 2012; 7(1):e30093. PMID:
22295072.

25. Johns LE, Ferguson KK, Meeker JD. Relationships between urinary phthalate metabolite and bisphenol a concentrations and vitamin D levels in U.S. adults: National Health and Nutrition Examination Survey (NHANES), 2005–2010. J Clin Endocrinol Metab. 2016; 101(11):4062–4069. PMID:
27648964.

26. Johns LE, Ferguson KK, Cantonwine DE, McElrath TF, Mukherjee B, Meeker JD. Urinary BPA and phthalate metabolite concentrations and plasma vitamin D levels in pregnant women: a repeated measures analysis. Environ Health Perspect. 2017; 125(8):087026. PMID:
28934718.

27. Kassi EN, Stavropoulos S, Kokkoris P, Galanos A, Moutsatsou P, Dimas C, et al. Smoking is a significant determinant of low serum vitamin D in young and middle-aged healthy males. Hormones (Athens). 2015; 14(2):245–250. PMID:
25402376.

28. Feizabad E, Hossein-Nezhad A, Maghbooli Z, Ramezani M, Hashemian R, Moattari S. Impact of air pollution on vitamin D deficiency and bone health in adolescents. Arch Osteoporos. 2017; 12(1):34. PMID:
28378273.

29. Barrea L, Savastano S, Di Somma C, Savanelli MC, Nappi F, Albanese L, et al. Low serum vitamin D-status, air pollution and obesity: a dangerous liaison. Rev Endocr Metab Disord. 2017; 18(2):207–214. PMID:
27645613.

30. Slominski AT, Janjetovic Z, Fuller BE, Zmijewski MA, Tuckey RC, Nguyen MN, et al. Products of vitamin D3 or 7-dehydrocholesterol metabolism by cytochrome P450scc show anti-leukemia effects, having low or absent calcemic activity. PLoS One. 2010; 5(3):e9907. PMID:
20360850.

31. Tuckey RC, Li W, Ma D, Cheng CY, Wang KM, Kim TK, et al. CYP27A1 acts on the pre-vitamin D3 photoproduct, lumisterol, producing biologically active hydroxy-metabolites. J Steroid Biochem Mol Biol. 2018; 181:1–10. PMID:
29452159.

32. Køster B, Søndergaard J, Nielsen JB, Allen M, Olsen A, Bentzen J. The validated sun exposure questionnaire: association of objective and subjective measures of sun exposure in a Danish population-based sample. Br J Dermatol. 2017; 176(2):446–456. PMID:
27412948.

33. Køster B, Søndergaard J, Nielsen JB, Olsen A, Bentzen J. Reliability and consistency of a validated sun exposure questionnaire in a population-based Danish sample. Prev Med Rep. 2018; 10:43–48. PMID:
29552457.

34. Wang TJ, Zhang F, Richards JB, Kestenbaum B, van Meurs JB, Berry D, et al. Common genetic determinants of vitamin D insufficiency: a genome-wide association study. Lancet. 2010; 376(9736):180–188. PMID:
20541252.
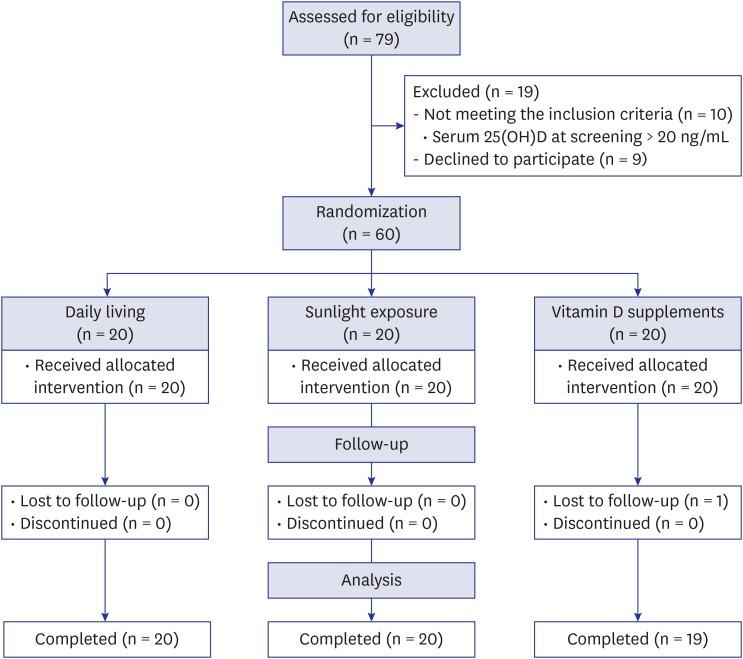
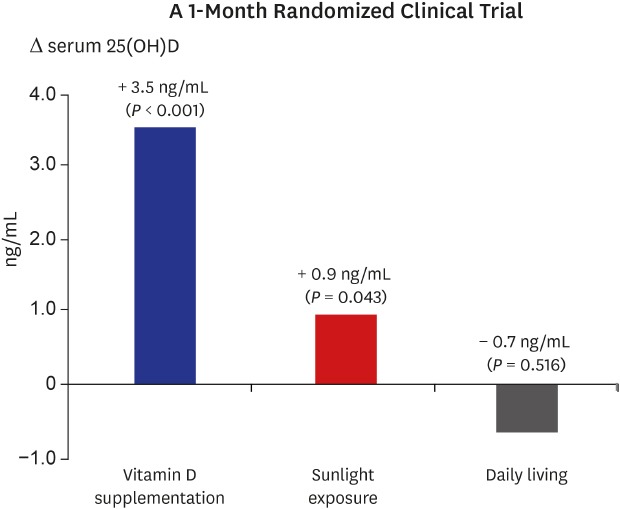
 ) indicates change in each individual's level of vitamin D; thick solid line (
) indicates change in each individual's level of vitamin D; thick solid line ( ) indicates the mean change in 25(OH)D level of the group; dotted line (
) indicates the mean change in 25(OH)D level of the group; dotted line ( ) indicates the cutoff value (20 ng/mL) of vitamin D insufficiency. P value for differences between baseline and follow-up were calculated using the Wilcoxon signed-rank test.
) indicates the cutoff value (20 ng/mL) of vitamin D insufficiency. P value for differences between baseline and follow-up were calculated using the Wilcoxon signed-rank test.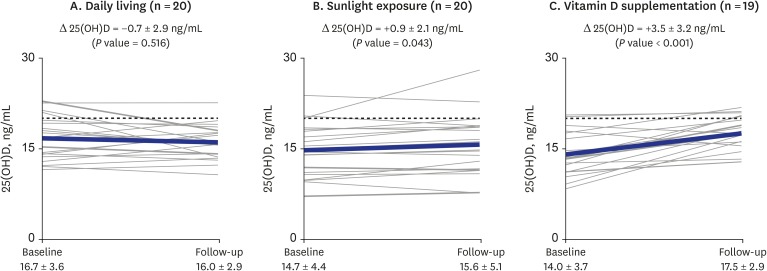
 ) indicates the change in each individual's level of vitamin D; thick solid line (
) indicates the change in each individual's level of vitamin D; thick solid line ( ) indicates the mean change in 1,25(OH)2D level of the group. P value for differences between baseline and follow-up were calculated using the Wilcoxon signed-rank test.
) indicates the mean change in 1,25(OH)2D level of the group. P value for differences between baseline and follow-up were calculated using the Wilcoxon signed-rank test.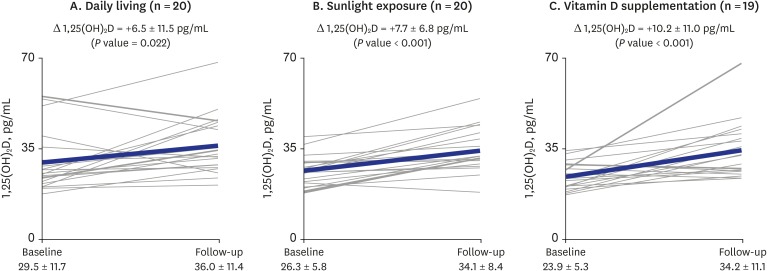
 ) indicates the change in each individual's level of vitamin D; thick solid line (
) indicates the change in each individual's level of vitamin D; thick solid line ( ) indicates the mean change in PTH level of the group. P value for differences between baseline and follow-up were calculated using the Wilcoxon signed-rank test.
) indicates the mean change in PTH level of the group. P value for differences between baseline and follow-up were calculated using the Wilcoxon signed-rank test.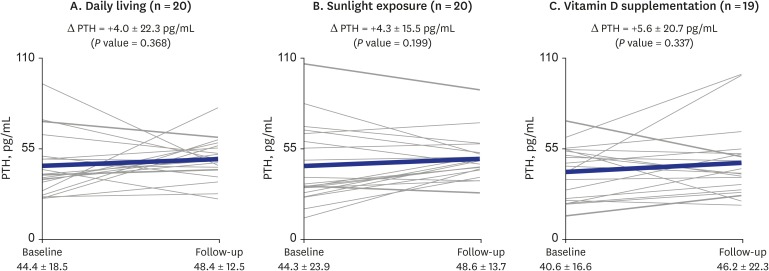




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download