Abstract
Immune checkpoint inhibitors (ICIs) have shown remarkable benefit in the treatment of patients with non-small-cell lung cancer (NSCLC) and have emerged as an effective treatment option even in the first-line setting. ICIs can block inhibitory pathways that restrain the immune response against cancer, restoring and sustaining antitumor immunity. Currently, there are 4 PD-1/PD-L1 blocking agents available in clinics, and immunotherapy-based regimen alone or in combination with chemotherapy is now preferred option. Combination trials assessing combination of ICIs with chemotherapy, targeted therapy and other immunotherapy are ongoing. Controversies remain regarding the use of ICIs in targetable oncogene-addicted subpopulations, but their initial treatment recommendations remained unchanged, with specific tyrosine kinase inhibitors as the choice. For the majority of patients without targetable driver oncogenes, deciding between therapeutic options can be difficult due to lack of direct cross-comparison studies. There are continuous efforts to find predictive biomarkers to find those who respond better to ICIs. PD-L1 protein expressions by immunohistochemistry and tumor mutational burden have emerged as most well-validated biomarkers in multiple clinical trials. However, there still is a need to improve patient selection, and to establish the most effective concurrent or sequential combination therapies in different NSCLC clinical settings. In this review, we will introduce currently used ICIs in NSCLC and analyze most recent trials, and finally discuss how, when and for whom ICIs can be used to provide promising avenues for lung cancer treatment.
Go to : 
References
1. Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015; 373:1627–1639.

2. Herbst RS, Baas P, Kim DW, Felip E, Pérez-Gracia JL, Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016; 387:1540–1550.

3. Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, Gadgeel SM, Hida T, Kowalski DM, Dols MC, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017; 389:255–265.

4. Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Kurata T, Chiappori A, Lee KH, de Wit M, et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med. 2018; 379:2342–2350.

5. Faruki H, Mayhew GM, Serody JS, Hayes DN, Perou CM, Lai-Goldman M. Lung adenocarcinoma and squamous cell carcinoma gene expression subtypes demonstrate significant differences in tumor immune landscape. J Thorac Oncol. 2017; 12:943–953.

6. Seo JS, Kim A, Shin JY, Kim YT. Comprehensive analysis of the tumor immune micro-environment in non-small cell lung cancer for efficacy of checkpoint inhibitor. Sci Rep. 2018; 8:14576.

7. Anichini A, Tassi E, Grazia G, Mortarini R. The non-small cell lung cancer immune landscape: emerging complexity, prognostic relevance and prospective significance in the context of immunotherapy. Cancer Immunol Immunother. 2018; 67:1011–1022.

8. Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe S, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016; 375:1823–1833.

9. Taube JM, Klein A, Brahmer JR, Xu H, Pan X, Kim JH, Chen L, Pardoll DM, Topalian SL, Anders RA. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res. 2014; 20:5064–5074.

10. Mehnert JM, Monjazeb AM, Beerthuijzen JM, Collyar D, Rubinstein L, Harris LN. The challenge for development of valuable immuno-oncology biomarkers. Clin Cancer Res. 2017; 23:4970–4979.

11. Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, Lee W, Yuan J, Wong P, Ho TS, et al. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015; 348:124–128.

12. Peters S, Creelan B, Hellmann MD, Socinski MA, Reck M, Bhagavatheeswaran P, Chang H, Geese WJ, Paz-Ares L, Carbone DP. Abstract ct082: Impact of tumor mutation burden on the efficacy of first-line nivolumab in stage IV or recurrent non-small cell lung cancer: an exploratory analysis of checkmate 026. Cancer Res. 2017; 77:CT082.

13. Ramalingam SS, Hellmann MD, Awad MM, Borghaei H, Gainor J, Brahmer J, Spigel DR, Reck M, O'Byrne KJ, Paz-Ares L, et al. Abstract ct078: Tumor mutational burden (tmb) as a biomarker for clinical benefit from dual immune checkpoint blockade with nivolumab (nivo) + ipilimumab (ipi) in first-line (1l) non-small cell lung cancer (nsclc): Identification of tmb cutoff from checkmate 568. Cancer Res. 2018; 78:CT078.

14. Hellmann MD, Ciuleanu TE, Pluzanski A, Lee JS, Otterson GA, Audigier-Valette C, Minenza E, Linardou H, Burgers S, Salman P, et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med. 2018; 378:2093–2104.

15. Felip Font E, Gettinger SN, Burgio MA, Antonia SJ, Holgado E, Spigel DR, Arrieta O, Domine Gomez M, Aren Frontera O, Brahmer J, et al. Three-year follow-up from checkmate 017/057: Nivolumab versus docetaxel in patients with previously treated advanced non-small cell lung cancer (NSCLC). Ann Oncol. 2017; 28(Suppl 5):mdx380. 004.

16. Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015; 373:123–135.

17. Büttner R, Gosney JR, Skov BG, Adam J, Motoi N, Bloom KJ, Dietel M, Longshore JW, López-Ríos F, Penault-Llorca F, et al. Programmed death-ligand 1 immunohistochemistry testing: a review of analytical assays and clinical implementation in non-small-cell lung cancer. J Clin Oncol. 2017; 35:3867–3876.

18. Tsao MS, Kerr KM, Kockx M, Beasley MB, Borczuk AC, Botling J, Bubendorf L, Chirieac L, Chen G, Chou TY, et al. PD-L1 immunohistochemistry comparability study in real-life clinical samples: results of blueprint phase 2 project. J Thorac Oncol. 2018; 13:1302–1311.

19. Lopes GW, Kudaba I, Kowalski D, Cho BC, Castro G, et al. Pembrolizumab versus platinum-based chemotherapy as first-line therapy for advanced/metastatic nsclc with a PD-L1 tumor proportion score >=1%: open-label, phase 3 keynote-042 study. J Clin Oncol. 2018; 36:36.
20. Carbone DP, Reck M, Paz-Ares L, Creelan B, Horn L, Steins M, Felip E, van den Heuvel MM, Ciuleanu TE, Badin F, et al. First-line nivolumab in stage IV or recurrent non-small-cell lung cancer. N Engl J Med. 2017; 376:2415–2426.

21. Rizvi NA, Chul Cho B, Reinmuth N, Lee KH, Ahn MJ, Luft A, van den Heuvel M, Cobo M, Smolin A, Vicente D, et al. Lba6durvalumab with or without tremelimumab vs platinum-based chemotherapy as first-line treatment for metastatic non-small cell lung cancer: mystic. Ann Oncol. 2018; 29(Suppl 10):mdy511. 005.
22. Bracci L, Schiavoni G, Sistigu A, Belardelli F. Immune-based mechanisms of cytotoxic chemotherapy: implications for the design of novel and rationale-based combined treatments against cancer. Cell Death Differ. 2014; 21:15–25.

23. Zitvogel L, Galluzzi L, Smyth MJ, Kroemer G. Mechanism of action of conventional and targeted anticancer therapies: reinstating immunosurveillance. Immunity. 2013; 39:74–88.

24. Roselli M, Cereda V, di Bari MG, Formica V, Spila A, Jochems C, Farsaci B, Donahue R, Gulley JL, Schlom J, et al. Effects of conventional therapeutic interventions on the number and function of regulatory T cells. OncoImmunology. 2013; 2:e27025.

25. Wang Z, Till B, Gao Q. Chemotherapeutic agent-mediated elimination of myeloid-derived suppressor cells. OncoImmunology. 2017; 6:e1331807.

26. Gandhi L, Garassino MC. Pembrolizumab plus chemotherapy in lung cancer. N Engl J Med. 2018; 379:e18.

27. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology. Non-small cell lung cancer [Internet]. Available at. https://www.nccn.org/professionals/physician_gls/default.aspx. [accessed on 2019].
28. Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, Rodríguez-Abreu D, Moro-Sibilot D, Thomas CA, Barlesi F, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018; 378:2288–2301.

29. Papadimitrakopoulou VA, Cobo M, Bordoni R, Longeras PD, Szalai Z, Ursol G, Novello S, Orlandi F, Ball S, Goldschmidt J, et al. Impower132: PFS and safety results with 1l atezolizumab + carboplatin/cisplatin + pemetrexed in stage IV non-squamous NSCLC. Proceedings of the IASLC 19th World Conference on Lung Cancer. 2018;2018 September 23–26; Toronto, Canada. Aurora, CO: International Association for the Study of Lung Cancer;. 2018.
30. Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gümüş M, Mazières J, Hermes B, Çay Ş enler F, Csőszi T, Fülöp A, et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med. 2018; 379:2040–2051.

31. Jotte RM, Cappuzzo F, Vynnychenko I, Stroyakovskiy D, Abreu DR, Hussein MA, Soo RA, Conter HJ, Kozuki T, Silva C, et al. Impower131: primary PFS and safety analysis of a randomized phase III study of atezolizumab + carboplatin + paclitaxel or nab-paclitaxel vs carboplatin + nab-paclitaxel as 1L therapy in advanced squamous NSCLC. J Clin Oncol. 2018; 36:LBA9000.

32. Bristol-Meyers Squibb. Bristol-Myers Squibb to announce results for fourth quarter 2018 on January 24 [Internet]. Available at. https://news.bms.com/press-release/corporatefinancial-news/bristol-myers-squibb-announce-results-fourth-quarter-2018-janu.
33. Ready N, Hellmann MD, Awad MM, Otterson GA, Gutierrez M, Gainor JF, Borghaei H, Jolivet J, Horn L, Mates M, et al. First-line nivolumab plus ipilimumab in advanced non-small-cell lung cancer (checkmate 568): outcomes by programmed death ligand 1 and tumor mutational burden as biomarkers. J Clin Oncol. 2019; 37:992–1000.

34. Papadopoulos KP, Lakhani NJ, Johnson ML, Park H, Wang D, Yap TA, Dowlati A, Maki RG, Lynce F, Ulahannan SV, et al. First-in-human study of REGN3767 (R3767), a human LAG-3 monoclonal antibody (mAb), ± cemiplimab in patients (pts) with advanced malignancies. J Clin Oncol. 2019; 37:2508.

35. Mølhøj M, Crommer S, Brischwein K, Rau D, Sriskandarajah M, Hoffmann P, Kufer P, Hofmeister R, Baeuerle PA. CD19-/CD3-bispecific antibody of the BiTE class is far superior to tandem diabody with respect to redirected tumor cell lysis. Mol Immunol. 2007; 44:1935–1943.

36. Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004; 10:909–915.

37. Meric-Bernstam F, Sandhu SK, Hamid O, Spreafico A, Kasper S, Dummer R, Shimizu T, Steeghs N, Lewis N, Talluto CC, et al. Phase ib study of miw815 (ADU-S100) in combination with spartalizumab (PDR001) in patients (pts) with advanced/metastatic solid tumors or lymphomas. J Clin Oncol. 2019; 37:2507.

38. Ahn MJ, Yang J, Yu H, Saka H, Ramalingam S, Goto K, Kim SW, Yang L, Walding A, Oxnard GR. 136o: osimertinib combined with durvalumab in egfr-mutant non-small cell lung cancer: results from the tatton phase ib trial. J Thorac Oncol. 2016; 11:S115.

39. Kim DW, Gadgeel SM, Gettinger SN, Riely GJ, Oxnard GR, Mekhail T, Schmid P, Dowlati A, Heist RS, Wozniakm AJ, et al. Safety and clinical activity results from a phase ib study of alectinib plus atezolizumab in ALK+ advanced NSCLC (aNSCLC). J Clin Oncol. 2018; 36:9009.

40. Lisberg A, Cummings A, Goldman JW, Bornazyan K, Reese N, Wang T, Coluzzi P, Ledezma B, Mendenhall M, Hunt J, et al. A phase II study of pembrolizumab in EGFR-mutant, PD-L1+, tyrosine kinase inhibitor naive patients with advanced NSCLC. J Thorac Oncol. 2018; 13:1138–1145.

41. Allard B, Pommey S, Smyth MJ, Stagg J. Targeting CD73 enhances the antitumor activity of anti-PD-1 and anti-CTLA-4 mAbs. Clin Cancer Res. 2013; 19:5626–5635.

42. Gandara DR, Paul SM, Kowanetz M, Schleifman E, Zou W, Li Y, Rittmeyer A, Fehrenbacher L, Otto G, Malboeuf C, et al. Blood-based tumor mutational burden as a predictor of clinical benefit in non-small-cell lung cancer patients treated with atezolizumab. Nat Med. 2018; 24:1441–1448.

43. Kim ES, Velcheti V, Mekhail T, Leal TA, Dowell JE, Tsai ML, Dakhil CS, Stella P, Shen V, Hu S, et al. Primary efficacy results from b-F1RST, a prospective phase II trial evaluating blood-based tumour mutational burden (bTMB) as a predictive biomarker for atezolizumab (atezo) in 1L non-small cell lung cancer (NSCLC). Ann Oncol. 2018; 29(Suppl 8):29.

44. Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018; 378:158–168.

45. Das S, Johnson DB. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer. 2019; 7:306.

46. Haratani K, Hayashi H, Chiba Y, Kudo K, Yonesaka K, Kato R, Kaneda H, Hasegawa Y, Tanaka K, Takeda M, et al. Association of immune-related adverse events with nivolumab efficacy in non-small-cell lung cancer. JAMA Oncol. 2018; 4:374–378.

47. Teraoka S, Fujimoto D, Morimoto T, Kawachi H, Ito M, Sato Y, Nagata K, Nakagawa A, Otsuka K, Uehara K, et al. Early immune-related adverse events and association with outcome in advanced non-small cell lung cancer patients treated with nivolumab: a prospective cohort study. J Thorac Oncol. 2017; 12:1798–1805.

48. Puzanov I, Diab A, Abdallah K, Bingham CO 3rd, Brogdon C, Dadu R, Hamad L, Kim S, Lacouture ME, LeBoeuf NR, et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer. 2017; 5:95.

49. Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, Chau I, Ernstoff MS, Gardner JM, Ginex P, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2018; 36:1714–1768.

50. Champiat S, Lambotte O, Barreau E, Belkhir R, Berdelou A, Carbonnel F, Cauquil C, Chanson P, Collins M, Durrbach A, et al. Management of immune checkpoint blockade dysimmune toxicities: a collaborative position paper. Ann Oncol. 2016; 27:559–574.

51. Robert C, Ribas A, Hamid O, Daud A, Wolchok JD, Joshua AM, Hwu WJ, Weber JS, Gangadhar TC, Joseph RW, et al. Durable complete response after discontinuation of pembrolizumab in patients with metastatic melanoma. J Clin Oncol. 2018; 36:1668–1674.

52. Kim JY, Lee KH, Kang J, Borcoman E, Saada-Bouzid E, Kronbichler A, Hong SH, de Rezende LF, Ogino S, Keum N, et al. Hyperprogressive disease during anti-PD-1 (PDCD1)/PD-L1 (CD274) therapy: a systematic review and meta-analysis. Cancers (Basel). 2019; 11:11.
Go to : 
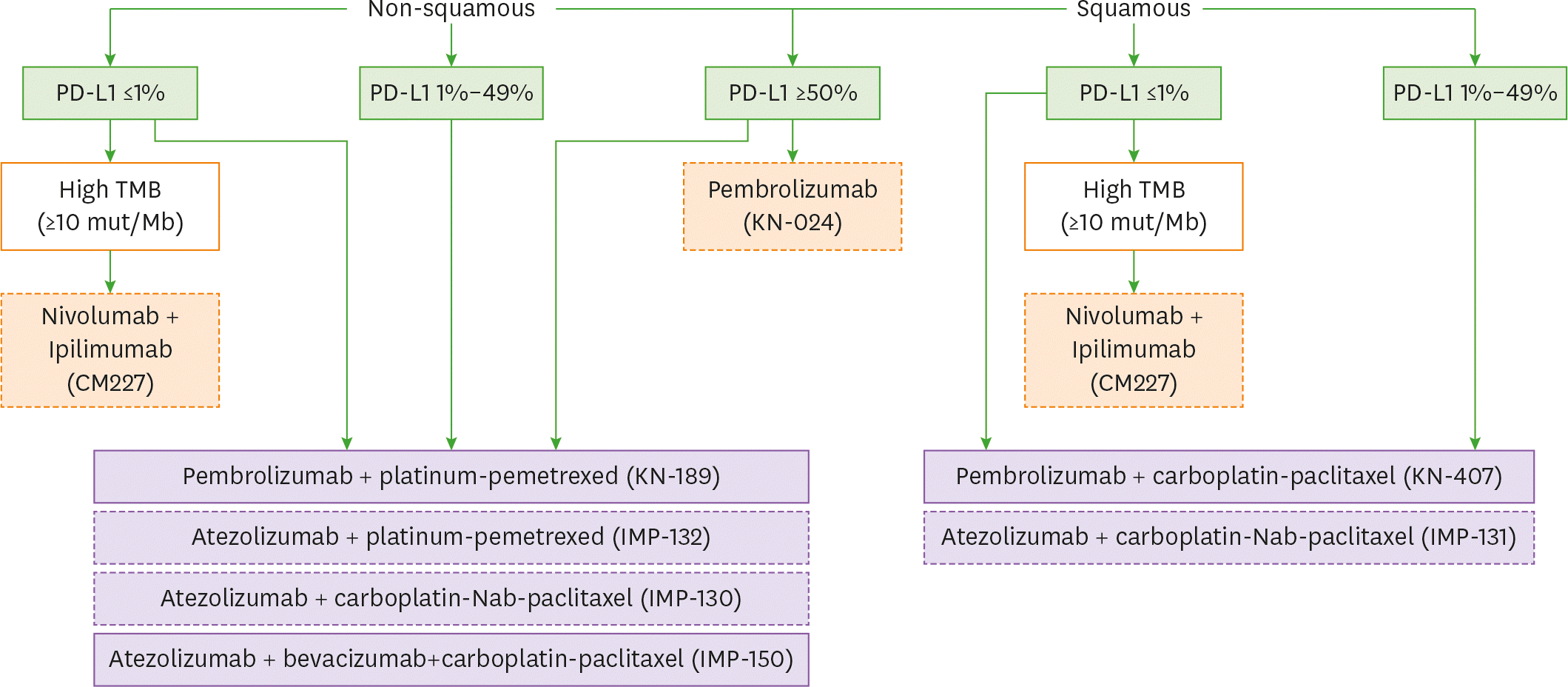 | Figure 1.First-line treatment algorithm. Dashed boxes indicate treatments which did not receive approval from regulatory agencies yet. TMB, tumor mutational burden. |
Table 1.
Pivotal studies of ICIs in advanced NSCLC




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download