Abstract
Chronic inflammatory airway diseases, such as chronic rhinosinusitis, chronic obstructive pulmonary disease, and asthma, are associated with excessive mucus production. Hence, the regulation of mucus production is important for the treatment of upper and lower airway diseases. Eupatilin is a pharmacologically active ingredient obtained from Artemisia asiatica Nakai (Asteraceae) and exerts potent anti-inflammatory, anti-allergic, and anti-tumor activities. In the present study, we investigated the effect of eupatilin on phorbol 12-myristate 13-acetate (PMA)-induced MUC5AC and MUC5B expression in human airway epithelial cells. We found that eupatilin treatment significantly inhibited PMA-induced mucus secretion in PAS staining. In addition, qRT-PCR results showed that eupatilin dose-dependently decreased the mRNA expression of MUC5AC in human airway epithelial cells. Western blot and immunofluorescence assay also showed that PMA-induced protein expression of MUC5AC was inhibited by eupatilin treatment. Finally, we investigated MAPKs activity after stimulation with PMA using western blot analysis in human airway epithelial cells. The results showed that eupatilin downregulated the levels of phosphorylated p38, ERK, and JNK. In summary, the anti-inflammatory activities of eupatilin, characterized as the suppression of MUC5AC expression and secretion in human airway epithelial cells, were found to be associated with the inhibition of p38/ERK/JNK MAPKs signaling pathway of MUC5AC secretion.
In human airways, mucus secretion plays a key role in protecting the upper airway tracts and lungs from the external environmental injury, such as pathogens, particles, and toxic chemicals [1]. The mucus layer acts as a protective barrier by entrapping inhaled foreign particles and bacteria, and clearing them from the airway by ciliary movement. In patients with chronic sinusitis, asthma, or chronic obstructive pulmonary disease (COPD), hypersecretion of airway mucus is a major feature [2]. Excessive mucus secretion leads to increase in the morbidity and mortality of the affected patients. Hence, regulation of mucus production is important in the treatment of upper and lower airway diseases.
Mucins, the major constituents of mucus, are responsible for the physical and biological properties of mucus [3]. The mucins are large glycosylated proteins and are produced by both goblet cells in the surface epithelium and mucous cells in the submucosal gland in the airway. To date, 21 or more subfamily mucin genes have been reported. Among mucin genes, MUC5AC and MUC5B encode the predominant secretory mucins present in inflammatory airway disease, and thus, many studies have described the expression of MUC5AC and MUC5B and their regulation as a therapeutic target for lung inflammatory disorders, such as bronchial asthma and airway infections [45]. Therefore, it is important to elucidate the potential of regulating the excessive secretion and production of mucin by the natural effective compounds isolated from various medicinal plants.
Eupatilin (5,7-dihydroxy-3′,4′,6′-trimethoxyflavone) is a pharmacologically active ingredient isolated from Artemisia asiatica Nakai (Asteraceae) and exerts potent anti-inflammatory, anti-allergic, and anti-tumor activities in several types of human cell lines [678]. However, the effects of eupatilin on mucin secretion in inflammatory airway epithelial cells have not yet been reported. Therefore, first, we investigated phorbol 12-myristate 13-acetate (PMA)-induced MUC5AC and MUC5B mucin gene expression in human airway cell line, NCI-H292. Second, we evaluated the effect of eupatilin on mucin expression and its signaling pathways in human airway epithelial cells.
Eupatilin and PMA were obtained from Sigma (St. Louis, MO, USA). RPMI-1640 medium, fatal bovine serum (FBS), penicillin, and streptomycin were purchased from Gibco (Thermo Fisher Scientific, Waltham, MA, USA). TRIzol reagent was obtained from Life Technologies (Carlsbad, CA, USA). For cDNA synthesis, SuperScript II Reverse Transcriptase was purchased from Thermo Scientific (Thermo Fisher Scientific, Vilnius, Lithuania). A monoclonal b-actin anti-body was obtained from Sigma. Antibodies for phospho-ERK-1/2 (p-ERK), total ERK-1/2 (ERK), phospho-p38 (p-p38), total p38 (p38), phospho-JNK (p-JNK), and total JNK (JNK) were obtained from Cell Signaling Technology Inc. (Beverly, MA, USA). Anti-MUC5AC antibody (SC-21701 AF488) was obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Donkey anti-Rabbit and anti-Mouse immunoglobulin secondary antibodies were obtained from Enzo Life Sciences (Farmingdale, NY, USA). The study protocol was approved by the institutional review board of Wonkwang University Hospital (IRB No. 2019-01-028-001). Informed consent was confirmed by the IRB.
NCI-H292 human airway epithelial cells (purchased from Korean Cell Line Bank, Seoul, Korea) were seeded in each plate and cultured in 2 ml of RPMI-1640 containing 10% FBS. The cells were seeded in wells of a 6-well plate at 1 × 105 cells/well and were grown at 37℃ under 5% CO2. For serum starvation, 70%–80% confluent cells were washed three times with unsupplemented RPMI-1640 and re-cultured in RPMI-1640 supplemented with 0.5% FBS for 24 h. After 24 h of serum starvation, the cells were pre-treated with varying concentrations of eupatilin for 1 h and were stimulated with PMA for each time.
Cell viability was measured using a Cell viability assay kit (CCK-8; Dojindo, Kumamoto, Japan), according to the manufacturer's instructions. Cells were seeded in a 96-well plate at a density of 5 × 103 cells/well and incubated for 24 h. Cells were further incubated with vehicle (DMSO) or various concentrations of eupatilin for 24 h, and then, added to each well for 4 h and optical density was measured at 450 nm representing cell viability.
Total RNA was extracted from NCI-H292 cells using TRIzol reagent, according to the manufacturer's instructions. cDNA was synthesized from 1 µg of total RNA. qRT-PCR was conducted in 20 ml reaction mixture, containing 10 ml SYBR Green Premix (Bioneer Co., Daejeon, Korea), 10 pmol forward primer, 10 pmol reverse primer, and 1 mg cDNA. The following primers were used for the real-time RT-PCR: GAPDH forward: 5′AATTCCATGGCACCGTCAAG3′; GAPDH reverse: 5′ATCGCCCCACTTGATTTTGG3′; MUC5AC forward: 5′TCAGCCCCGAGTTCAAGG3′; MUC5AC reverse: 5′TTCCCAAACTCCAGCACGTC3′; MUC5B forward: 5′AGTCCATTTGCTGACCCCAC3′; and MUC5B reverse: 5′GGATGGTCGTGTTGATGCG3′. The human GAPDH gene was used as the internal control. The amplification parameters consisted of initial denaturation at 95℃ for 5 min, and 40 cycles of 3-step PCR: denaturation at 95℃ for 1 min, annealing at 60℃ for 30 sec, and extension at 72℃ for 1 min. The data was normalized against GAPDH levels, and presented as the mean fold change. Relative gene expression was calculated by evaluating qRT-PCR data using the 2−ΔΔCt method.
After treatments, the cells were washed three times with cold phosphate-buffered saline (PBS) and lysed with lysis buffer containing 50 mM Tris-HCl, 150 mM NaCl, 5 mM EDTA, 1% Triton X-100, 1 mM sodium vanadate, 1% deoxycholate, and protease inhibitors. The protein content in the extract was determined using a Bio-Rad DC Protein Assay Kit (Bio-Rad Laboratories Inc., Hercules, CA, USA). Equal amounts of proteins (30 µg) were run on 6%–10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels, and transferred to polyvinylidene difluoride membranes (Millipore, Bedford, MA, USA). The membranes were blocked with 5% nonfat milk in Tris-buffered saline supplemented with 0.1% Tween 20 (TBST) for 1 h, washed with TBST, and incubated with primary antibodies for 2 h. The membranes were incubated for 1 h with appropriate secondary antibodies. After washing with TBST, the immunoreactivity was detected using a Western Chemiluminescent HRP Substrate Kit (Millipore, Billerica, MA, USA).
NCI-H292 cells were seeded at a density of 2 × 105 cells/well onto 19 mm glass cover slips placed in a 12-well plate and serum-starved for 24 h. After 24 h starvation with RPMI-1640 media containing 0.5% FBS, cells were treated with eupatilin for 1 h, and then, stimulated with PMA for 1 h. Cells were fixed with 4% formalin in PBS, were permeabilized with 0.1% Triton X-100. The cells were blocked with 2.5% bovine serum albumin in PBS for 1 h, and after washing, the cells were incubated with MUC5AC (Alex 488) antibody diluted 1:100 in PBS containing 1.5% bovine serum albumin (BSA) overnight at 4℃. Nuclei were counterstained with DAPI. Images were captured using an Olympus FV1000 confocal laser microscope.
Mucins were characterized using the PAS staining kit (American Master Tech, Lodi, CA, USA). Cultured cells were fixed with 10% formalin in PBS for 10 min and rinsed in distilled water. The cells stained with 0.5% periodic acid for 7 min and washed three times with distilled water (DW). Then, the cells were stained with optimized Schiff's solution for 15 min and washed three times with DW. Counterstain was accomplished with modified Mayer's Hematoxylin solution for 2 min, and PAS staining was used to visualize the mucous glycoprotein conjugates.
All experiments were conducted at least three times and data was expressed as the mean ± standard deviation (SD). The data were analyzed with GraphPad Prism version 5 software (GraphPad software, Inc., San Diego, CA, USA). Statistical differences were analyzed using one-way ANOVAs followed by Tukey's post-hoc test. A p-value < 0.05 was considered statistically significant.
The chemical structure of eupatilin is shown in Fig. 1A. It is an O-methylated flavone with anti-oxidative, anti-inflammatory, anti-cancer properties [7]. Initially, we evaluated the viability effect of various concentrations of eupatilin on NCI-H292 human airway epithelial cells via Cell viability assay. We found that eupatilin had no effect on cell proliferation at concentrations up to 2 µM (Fig. 1B). Therefore, we next studied the effect of 2 µM eupatilin on inflammatory response, such as expression and secretion of MUC5AC or MUC5B gene in airway epithelial cells.
NCI-H292 cells were incubated with various concentrations of PMA (0, 1, 5, 10, and 20 nM) for 8 h. The mRNA expressions of MUC5AC and MUC5B mucin glycoproteins were increased by PMA dose-dependently and were significantly induced at PMA concentration of 20 nM. Therefore, the dose of 20 nM of PMA was used for inflammatory stimulation of NCI-H292 human airway epithelial cells (Fig. 2A, B). We examined whether eupatilin regulates the expression of mucin protein that is expressed in the mucus layers of human airway and are predominant components of airway mucus related diseases, such as chronic rhinosinusitis and asthma. NCI-H292 cells were incubated with 20 nM PMA and various concentrations of eupatilin (0, 0.1, 0.5, 1, and 2 µM) for 8 h. PMA induced MUC5AC and MUC5B mRNA expressions. Moreover, eupatilin specifically inhibited PMA-induced MUC5AC expressions in a dose dependent manner (Fig. 2C, D).
To investigate whether eupatilin affected PMA-induced MUC5AC protein expression, we examined the protein expression level of MUC5AC after PMA treatment. PMA stimulation induced the expression of MUC5AC. However, MUC5AC expression was significantly decreased after eupatilin treatment (Fig. 3A). We next investigated the effect of eupatilin on MUC5AC protein expression using immunofluorescence assay. Immunofluorescence assay also indicated that PMA treatment induced MUC5AC expression, but eupatilin treatment reduced MUC5AC expression in the cytoplasm (Fig. 3B).
MUC5AC expression is influenced by activities of various proteins, such as ERK, JNK, and p38 MAPK, which are important in signal transduction pathway of mucin [910]. To elucidate the mechanism by which PMA-induced MUC5AC gene expression was suppressed by eupatilin, we examined which MAPKs were activated in NCI-H292 cells after PMA stimulation. PMA activated the phosphorylation of several signal transducers, including ERK, p38, and JNK, involved in MUC5AC gene expression. The phosphorylation of ERK, JNK, and p38 was significantly inhibited by eupatilin (Fig. 4). Taken together, we concluded that eupatilin-mediated inhibition of MAPKs reduces MUC5AC glycoprotein expression.
PMA promotes intracellular mucin release in respiratory epithelial cells and glycoproteins, such as mucin proteins, could be visualized with PAS staining. The levels of PAS-stained glycoproteins were increased in cells after PMA stimulation compared with control. The increase in PAS-stained mucin levels by PMA stimulation was dose-dependently attenuated by various concentrations of eupatilin in NCI-H292 cells (Fig. 5).
Chronic inflammatory airway diseases, such as chronic rhinosinusitis, COPD, and asthma, are associated with excessive mucus production, which contributes to the morbidity and mortality of the patients. However, the mechanism of mucus overproduction has not yet been completely elucidated. In the past, most of the medication to remove excessive mucus from the airway was focused on attenuation of overproducing and hypersecreting mucus. However, regulation of mucin secretion and production has become an important approach to control the hypersecretion of airway mucus [1112]. Thus, we investigated the effects of eupatilin on PMA-induced MUC5AC and MUC5B mucin gene expression and production in NCI-H292 cells, a human pulmonary mucoepidermoid cell line, often used for elucidating mechanisms involved in airway mucin production and gene expression [1314].
The mucins present in inflammatory airway disease are induced by various inflammatory mediators, such as epidermal growth factor (EGF), tumor necrosis factor (TNF)-α, lipopolysaccaride (LPS), and PMA. It has been reported that epidermal growth factor regulates MUC5AC gene expression by binding of EGF to its receptor and subsequent activation of MAPK cascade [14]. In addition, TNF-α has been reported to provoke secretion and gene expression of pulmonary mucin [1516]. Thus, TNF-α stimulated MUC5AC gene expression in normal human airway epithelial cells. TNF-α-converting enzyme mediates MUC5AC mucin expression in cultured human airway epithelial cells [13]. LPS has been shown to upregulate the levels of expression of MUC5AC mRNA in HT-29 goblet cells in vitro [17]. Several proinflammatory cytokines, namely, interleukin (IL)-1β, IL-6, and IL-17, upregulate MUC5AC expression in airway epithelial cells. PMA is an inflammatory stimulant that regulates gene transcription, cell growth, and differentiation [918]. It may also induce the gene expression of pro-inflammatory cytokines, including TNF-α [51014]. Especially, PMA is involved in the secretion of mucins and is used as a protein kinase C (PKC) activator and provoked the expressions of mucin genes, including MUC5AC and MUC5B in human airway cells. MUC5AC is a major component of gel forming mucins in airway system and is associated with regulation of TGF-α release and EGFR activation via Ras, ERK, and Sp1-dependent mechanisms under PMA stimulation [1219]. In this study, PMA was found to induce MUC5AC and MUC5B mRNA expressions in a dose dependent manner and the upregulated mucin gene expression of only MUC5AC was inhibited by eupatilin treatment (Fig. 2). In addition, the PMA-induced MUC5AC protein expression and immunofluorescence intensity was also significantly reduced by eupatilin treatment (Fig. 3). Eupatilin suppressed PMA-induced production of mucus in a dose dependent manner (Fig. 5). Therefore, the hypersecretion of mucus by PMA stimulation was reduced by eupatilin and which was shown its potential effects as a modulator in appropriate maintaining the basal levels of MUC5AC for the defense mechanism against external stimuli in innate immunity.
Eupatilin exerts potent anti-inflammatory, anti-allergic, and anti-tumor activities in several types of cells or human cell lines [67820]. Eupatilin was shown to induce cell cycle arrest in H-ras-transformed human mammary epithelial (MCF10A-ras) cells [7]. Anti-proliferative effect of eupatilin in MCF10A-ras cells was associated with inhibition of ERK1/2 activation. Anti-inflammatory activities of eupatilin induce the suppression of eotaxin expression in bronchial epithelial cells [21]. Eupatilin was also shown to inhibit the signaling of MAPK-IKK-NF-κB, leading to inhibition of eosinophil migration. In PMA plus calcium inophore A23187-stimulated and anaphylactic shock model, eupatilin exhibits anti-allergic properties and significantly suppresses the production of pro-inflammatory cytokines, such as IL-1β, TNF-α, and IL-6 by inhibiting NF-κB activation and phosphorylation of p38, ERK, and JNK MAPKs [20].
In the present study, we found that eupatilin treatment significantly inhibited PMA-induced MUC5AC secretion in NCI-H292 cells (Figs. 2 and 3), which corroborates with suppression of phosphorylation of p38, ERK1/2, and JNK (Fig. 4). It is well known that the activation of the MAPK signaling is speculated to be most important in transmitting inflammatory signals from the cell surface to the nucleus. The cascade is a pivotal step in the regulation of cellular responses, such as cell growth, proliferation, differentiation, and secretion in mammals [22]. After being triggered by various growth factors, cytokines and other stress-inducible agents, a signal is transduced via the ERK, p-38, and JNK MAPKs cascade. PMA induces MUC5AC expression via an EGFR-related signaling pathway involving ERK and Sp1 activation [12]. IL-1β and TNF-α induce MUC5AC gene expression by regulating the activation of mitogen- and stress-activated protein kinase 1 (MAK1) and CREB. Non-typeable Haemophilus influenzae was introduced to regulate MUC5AC transcription via p-38 MAP kinase in human epithelial cells [2023]. MUC5AC also undergoes transcriptional upregulation in response to tobacco smoke, which activates JNK via a Src-dependent, EGFR-independent signaling cascade [24]. In the present study, the phosphorylation and activation of three major MAPKs, p38, ERK1/2, and JNK, showed the maximum expression levels at 30 min after initiation of treatment. The phosphorylation of MAPKs was strongly inhibited by eupatilin, after treatment with PMA (Fig. 4).
Eupatilin suppressed PMA-induced expression of MUC5AC, but not of MUC5B. The possible mechanisms of that can be explained as follows. In normal human airway, MUC5AC mainly expressed by surface goblet epithelial cells, whereas MUC5B is predominantly expressed by mucous cells of submucosal glands. PMA up-regulates MUC5AC and MUC5B expression in human airway epithelial cells. PMA induced MUC5AC expression via EGFR, Ras, Raf, ERK, and Sp1 signaling pathway while MUC5B was regulated by PKCδ, Ras, and JNK/p38 MAPK-dependent pathway but not by EGFR/ERK-dependent pathway. This specific variation on MUC5AC and MUC5B expression by eupatilin may be due to differences in expression region and mechanism between the two genes and the characteristics of NCI-H292 cells.
In summary, our results suggested that eupatilin might inhibit PMA-induced MUC5AC expression and secretion via p38, ERK, and JNK MAPKs signaling pathways in NCI-H292 cells. These findings provided the first evidence that eupatilin might be an important therapeutic compound to treat mucus hypersecretion diseases, such as COPD and asthma.
Figures and Tables
Fig. 1
The structure and effect of eupatilin on NCI-H292 cell viability.
(A) The chemical structure of eupatilin. (B) NCI-H292 cells were treated with indicated doses of eupatilin or vehicle (DMSO, control). Each well was treated with cell viability assay solution for 4 h and the viability of cells was determined with absorbance at 450 nm. ns, not significant.
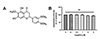
Fig. 2
Effects of eupatilin on the phorbol 12-myristate 13-acetate (PMA)-induced mRNA expression levels of MUC5AC and MUC5B in NCI-H292 cells.
(A, B) NCI-H292 cells were stimulated with indicated doses of PMA or vehicle (DMSO, control) for 8 h. The mRNA expression levels of PMA-stimulated MUC5AC (A) and MUC5B (B) was determined via real-time RT-PCR. (C, D) NCI-H292 cells were pre-treated with varying concentrations of eupatilin for 1 h, and then, stimulated with 20 nM PMA for 8 h. MUC5AC (C) and MUC5B (D) gene expressions were determined by real-time RT-PCR. ns, not significant compared with PMA alone; *p < 0.05, **p < 0.01 compared with control; ##p < 0.01 compared with PMA alone.

Fig. 3
Effects of eupatilin on the phorbol 12-myristate 13-acetate (PMA)-induced protein expression level of MUC5AC in NCI-H292 cells.
(A) Serum-starved NCI-H292 cells were pre-treated with or without eupatilin for 1 h, and then, stimulated with PMA for indicated time periods. The protein expression level of MUC5AC was determined by western blot analysis. (B) MUC5AC protein expression in PMA-induced NCI-H292 cells stimulated with or without eupatilin was visualized by immunofluorescence staining. Cells were stained with anti-MUC5AC antibody (Alex 488, green) and DAPI (nuclear stain, blue). Cells were stained with anti-MUC5AC antibody (Alex 488, green) and DAPI (nuclear stain, blue) and were visualized with a 40× objective.
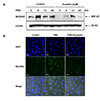
Fig. 4
Effects of eupatilin on the phorbol 12-myristate 13-acetate (PMA)-induced MAPK signal in NCI-H292 cells.
Serum-starved NCI-H292 cells were pre-treated with or without eupatilin for 1 h, and then, stimulated with PMA for indicated time periods. The protein levels of ERK, p-38, and JNK were analyzed by western blotting.
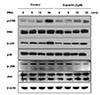
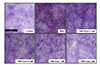
Fig. 5
Effect of eupatilin on the phorbol 12-myristate 13-acetate (PMA)-induced the production of mucus.
Serum-starved NCI-H292 cells were pre-treated with or without eupatilin and stimulated with PMA. The cells were stained by PAS and incorporated with hematoxylin counterstaining the nuclei. The images were acquired with a 10× objective. EUP, eupatilin.
Notes
References
1. Bae CH, Jeon BS, Choi YS, Song SY, Kim YD. Delphinidin inhibits LPS-induced MUC8 and MUC5B expression through toll-like receptor 4-mediated ERK1/2 and p38 MAPK in human airway epithelial cells. Clin Exp Otorhinolaryngol. 2014; 7:198–204.

2. Aikawa T, Shimura S, Sasaki H, Ebina M, Takishima T. Marked goblet cell hyperplasia with mucus accumulation in the airways of patients who died of severe acute asthma attack. Chest. 1992; 101:916–921.


3. Ali Mel-S. Nasosinus mucin expression in normal and inflammatory conditions. Curr Opin Allergy Clin Immunol. 2009; 9:10–15.


4. Fahy JV. Goblet cell and mucin gene abnormalities in asthma. Chest. 2002; 122:6 Suppl. 320S–326S.

6. Jung J, Ko SH, Yoo do Y, Lee JY, Kim YJ, Choi SM, Kang KK, Yoon HJ, Kim H, Youn J, Kim JM. 5,7-Dihydroxy-3,4,6-trimethoxyflavone inhibits intercellular adhesion molecule 1 and vascular cell adhesion molecule 1 via the Akt and nuclear factor-κB-dependent pathway, leading to suppression of adhesion of monocytes and eosinophils to bronchial epithelial cells. Immunology. 2012; 137:98–113.


7. Kim DH, Na HK, Oh TY, Kim WB, Surh YJ. Eupatilin, a pharmacologically active flavone derived from Artemisia plants, induces cell cycle arrest in ras-transformed human mammary epithelial cells. Biochem Pharmacol. 2004; 68:1081–1087.


8. Seo HJ, Park KK, Han SS, Chung WY, Son MW, Kim WB, Surh YJ. Inhibitory effects of the standardized extract (DA-9601) of Artemisia asiatica Nakai on phorbol ester-induced ornithine decarboxylase activity, papilloma formation, cyclooxygenase-2 expression, inducible nitric oxide synthase expression and nuclear transcription factor kappa B activation in mouse skin. Int J Cancer. 2002; 100:456–462.

9. Hewson CA, Edbrooke MR, Johnston SL. PMA induces the MUC5AC respiratory mucin in human bronchial epithelial cells, via PKC, EGF/TGF-alpha, Ras/Raf, MEK, ERK and Sp1-dependent mechanisms. J Mol Biol. 2004; 344:683–695.

10. Yuan-Chen Wu D, Wu R, Reddy SP, Lee YC, Chang MM. Distinctive epidermal growth factor receptor/extracellular regulated kinase-independent and -dependent signaling pathways in the induction of airway mucin 5B and mucin 5AC expression by phorbol 12-myristate 13-acetate. Am J Pathol. 2007; 170:20–32.



11. Kim JO, Sikder MA, Lee HJ, Rahman M, Kim JH, Chang GT, Lee CJ. Phorbol ester or epidermal growth-factor-induced MUC5AC mucin gene expression and production from airway epithelial cells are inhibited by apigenin and wogonin. Phytother Res. 2012; 26:1784–1788.


12. Park SH, Lee HJ, Ryu J, Son KH, Kwon SY, Lee SK, Kim YS, Hong JH, Seok JH, Lee CJ. Effects of ophiopogonin D and spicatoside A derived from Liriope Tuber on secretion and production of mucin from airway epithelial cells. Phytomedicine. 2014; 21:172–176.


13. Shao MX, Ueki IF, Nadel JA. Tumor necrosis factor alpha-converting enzyme mediates MUC5AC mucin expression in cultured human airway epithelial cells. Proc Natl Acad Sci U S A. 2003; 100:11618–11623.


14. Takeyama K, Dabbagh K, Lee HM, Agustí C, Lausier JA, Ueki IF, Grattan KM, Nadel JA. Epidermal growth factor system regulates mucin production in airways. Proc Natl Acad Sci U S A. 1999; 96:3081–3086.



15. Fischer BM, Rochelle LG, Voynow JA, Akley NJ, Adler KB. Tumor necrosis factor-alpha stimulates mucin secretion and cyclic GMP production by guinea pig tracheal epithelial cells in vitro. Am J Respir Cell Mol Biol. 1999; 20:413–422.

16. Song KS, Lee WJ, Chung KC, Koo JS, Yang EJ, Choi JY, Yoon JH. Interleukin-1 beta and tumor necrosis factor-alpha induce MUC5AC overexpression through a mechanism involving ERK/p38 mitogen-activated protein kinases-MSK1-CREB activation in human airway epithelial cells. J Biol Chem. 2003; 278:23243–23250.

17. Smirnova MG, Guo L, Birchall JP, Pearson JP. LPS up-regulates mucin and cytokine mRNA expression and stimulates mucin and cytokine secretion in goblet cells. Cell Immunol. 2003; 221:42–49.


18. Park SJ, Kang SY, Kim NS, Kim HM. Phosphatidylinositol 3-kinase regulates PMA-induced differentiation and superoxide production in HL-60 cells. Immunopharmacol Immunotoxicol. 2002; 24:211–226.


20. Song EH, Chung KS, Kang YM, Lee JH, Lee M, An HJ. Eupatilin suppresses the allergic inflammatory response in vitro and in vivo. Phytomedicine. 2018; 42:1–8.

21. Jeon JI, Ko SH, Kim YJ, Choi SM, Kang KK, Kim H, Yoon HJ, Kim JM. The flavone eupatilin inhibits eotaxin expression in an NF-κB-dependent and STAT6-independent manner. Scand J Immunol. 2015; 81:166–176.


22. Garrington TP, Johnson GL. Organization and regulation of mitogen-activated protein kinase signaling pathways. Curr Opin Cell Biol. 1999; 11:211–218.


23. Wang B, Lim DJ, Han J, Kim YS, Basbaum CB, Li JD. Novel cytoplasmic proteins of nontypeable Haemophilus influenzae upregulate human MUC5AC mucin transcription via a positive p38 mitogen-activated protein kinase pathway and a negative phosphoinositide 3-kinase-Akt pathway. J Biol Chem. 2002; 277:949–957.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download