Abstract
Ulcerative colitis (UC) is associated with intestinal immune imbalance and inflammatory response. Because dehydrolovastatin (DLVT), a derivative of lovastatin, has been recently shown to inhibit inflammation and relieve immune arthritis induced by chemical stimuli, we studied its effect and possible mechanism on UC induced by dextran sulfate sodium. The BALB/c mice were classified into six groups: normal control group, model group, DLVT high dose group, DLVT low dose group, salazosulfapyridine (SASP) group and lovastatin (LVT) group. The disease activity indices of UC and pathological changes were investigated. The myeloperoxidase (MPO) activity in colon tissue and inflammatory factors such as IL-6, IL-10, IL-17, and TNF-α in the serum were analyzed by ELISA, while the expression of NF-κB p65 protein in colon tissue was detected by immunohistochemistry and western blot. DLVT relieved the disease activity indices and pathological damage of the UC mice. Furthermore, DLVT significantly decreased MPO activity and improved the imbalance of inflammatory cytokines through inhibiting the expression of NF-κB p65. Meanwhile, the positive drug of SASP has a similar effect to DLVT, but the effect of DLVT in both decreasing IL-17, TNF-α, and increasing IL-10 was significantly stronger than that of SASP. These results suggest that DLVT may ameliorates the symptoms of UC.
Ulcerative colitis (UC) is a chronic, nonspecific and recurrent intestinal inflammatory disease. Its typical clinical symptoms include mucous purulent bloody stool, diarrhea and abdominal pain [1]. Many factors, such as immune abnormality, intestinal flora disorder and heredity, may be related to the occurrence and development of this disease [234]. In recent years, the incidence and mortality of UC worldwide have been increasing [5]. The pathological changes of the disease are complex and its clinical symptoms are diverse. If the patient has long disease duration, the patient has higher risk of colon cancer [6]. Therefore, UC is listed as one of the modern refractory diseases by World Health Organization [78]. The commonly used drugs for UC include amino salicylic acid, glucocorticoid, immunosuppressive and some traditional Chinese medicine, but the curative effect is not ideal [910].
Recent studies have gained a deeper understanding of the cellular and molecular mechanisms of the immune-inflammatory pathway, and it has been recognized that UC is an atypical autoimmune response. Besides immune cells, non-immune cells in the intestinal mucosa, such as epithelial cells, vascular endothelial cells and mesenchymal cells, also participate in the immune response and inflammatory process [11]. These cells interact with each other to release a variety of cytokines and inflammatory factors such as IL-1, IL-2, IL-6, IL-17, and TNF-α. The imbalance of inflammatory factors and anti-inflammatory factors leads to the continuous inflammation of intestinal mucosa, the impairment of barrier function and the unhealed ulcer [12]. In addition, the significant therapeutic effects of some biological agents such as monoclonal antibody against TNF-α based on the inflammatory response pathway also proved that intestinal mucosal immune factors always play an important role in the occurrence and development of UC [13]. On the other hand, it has been reported that statins have certain therapeutic effects on UC [1415]. This kind of drugs have the effect of inhibiting the adhesion and secretion of immune cells, reducing the plasma C-Reactive Protein and thus reducing the inflammatory response [16]. And this anti-inflammatory effect of statins, including lovastatin (LVT), is related to the decrease of the expression of NF-κB [1718].
Dehydrolovastatin (DLVT), a derivative of LVT, is a new member of the statin family in recent years. Previous studies in our laboratory have shown that DLVT has the effect of reducing blood lipid similar to LVT. Meanwhile, DLVT can not only dose-dependently suppress inflammation induced by chemical stimuli, but also inhibit experimental immune arthritis [1920]. Because DLVT has a similar chemical structure to LVT, we speculate that DLVT may also have some therapeutic effect on UC by inhibiting the expression of NF-κB and reducing the inflammatory response. Furthermore, this effect of DLVT has not been reported previously. Therefore, in this study, we investigated the effect and possible mechanism of DLVT on UC induced by 4% dextran sulfate sodium (DSS) in BALB/c mice.
Sixty adult male clean-level BALB/c mice (weighting 22–25 g, from Experimental Animal Center of Chongqing Medical University) were used for this study. Certification number is SCXK(yu)2014-0001. Mice were housed under standard conditions of a 12 h light/dark cycle, 20℃–26℃ and 40%–60% humidity. This study was approved by the Chengdu University of Traditional Chinese Medicine Ethics Review Committee (TCM-2016-312) and conducted in accordance with the United States National Institute of Health “Guide for the Care and Use of Laboratory Animals” (2010).
DLVT appears as milky fluffy coarse powder, slightly soluble in water. LVT was provided by Chongqing Daxin Pharmaceutical Co., LTD. (Chongqing, China). Salazosulfapyridine (SASP) was provided by Shanghai Fuda Pharmaceutical Co., LTD. (Shanghai, China) When the above drugs were applied, a certain amount was weighed with the electronic analytical balance. After being ground in the mortar, 0.5% sodium carboxymethylcellulose solution was used to prepare the uniform suspension for use.
DSS (molecular weight:36000-50000, MP Biomedicals, Aurora, OH, USA) was added at 4% concentration. The stool occult blood assay kit and the myeloperoxidase (MPO) assay kit were purchased from Nanjing Jiancheng Biological Engineering Institute (Nanjing, China). IL-6, IL-10, IL-17 and TNF-α ELISA kit were purchased from R&D Systems (Minneapolis, MN, USA). Mouse anti-NF-κB p65 monoclonal antibody was purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA). Immunohistochemical reagent and DAB color test kit were purchased from Wuhan Boshide Bioengineering Co., LTD. (Wuhan, China).
A2000S electronic analytical balance was provided by Sartorius Inc. (Goettingen, Germany); −80℃ ultra-low temperature freezer was provided by Binder Inc. (Tuttlingen, Germany); Rayto RT-6500 enzyme-mark analyzer was provided by Rayto Inc. (Shenzhen, China); BH-2 light microscope was provided by Olympus Inc. (Tokyo, Japan); Sigma-3k cryogenic centrifuge was provided by Sigma-Aldrich Inc. (St. Louis, MO, USA); UV-225 ultravioletvisible spectrophotometer was provided by Shimadzu Inc. (Kyoto, Japan).
After one week of adaptive feeding of sixty BALB/c mice, they were randomly divided into six groups (n = 10 for each group). That was normal control group, DSS model group, DLVT high dose treated group (33.6 mg/kg/d), DLVT low dose treated group (16.8 mg/kg/d), SASP treated group (250 mg/kg/d) and LVT treated group (16.8 mg/kg/d). Each drug-treated group was intragastrically administered with the corresponding drug once a day for 10 days. The normal control group and the model group were given an equal volume of 0.5% vehicle once a day for 10 days. On the third day after the administration, the mice were free to drink 4% DSS except for the normal control group [21], and the fresh DSS aqueous solution was changed every day until the end of the experiment.
From the fourth day of the experiment, the mice were weighed every two days, their activities, coat color, mental condition, diet and stool were observed, and the stool occult blood test was performed. According to the evaluation criteria of Disease Activity Index (DAI) of Cooper et al. [22], three indices, namely, percentage of weight loss, stool occult blood and stool characteristics, were used for scoring (Table 1). The sum of the above three scores was divided by 3, which was the DAI of each mouse.
At 24 h after the last administration, the mice were sacrificed by cervical dislocation and the blood was collected from the retro- orbital blood sinus. The whole colon was cut from the anus to the ileocecal area and the length was measured. The longitudinal section was carefully cut along the edge of the mesentery. After the contents were cleaned and washed with normal saline, excess water was removed by filter paper. Middle colon tissue was cut 1.0 cm from the end of the anus, fixed with 4% neutral formaldehyde, then embedded in paraffin before being cut into 4 mm sections, and subjected to histological examination by hematoxylin and eosin staining. Histopathological changes were examined by light microscopy at high-power visual fields (20×) and histological injury score was performed [2324]. The scoring criteria were as follows: (1) Exudation scoring standard of inflammatory cells: 0 point, there were very few inflammatory cells in the lamina propria of the mucosa; 1 point, there were more inflammatory cells in the lamina propria of the mucosa; 2 points, inflammatory cells were spread to the submucosa; 3 points, inflammatory cells were exuded in the whole layer; (2) Tissue damage scoring standard: 0 point, no mucosal damage; 1 point, non-continuous mucosal epithelial damage; 2 points, superficial mucosal erosion; 3 points, extensive mucosal damage and deep expansion into the colonic wall. The scores of inflammatory cell exudation and tissue damage were added together, and the histological injury score (1–6 points) was calculated. All histological evaluations were done by skilled pathologist who didn't know the groups information.
Colonic tissue was rinsed in cold normal saline, then dried with filter paper. After accurate weighing, it was immediately cut with an ophthalmic scissors, placed in a glass homogenizer in an ice bath, and then homogenized by adding 0.86% saline. 10% tissue homogenate was prepared by adding homogenate medium with a weight to volume ratio of 1:9. MPO is an enzyme mainly found in azeotropic granules of neutrophils, whose activity reflects the number and activity of neutrophil infiltration, and is an indicator to evaluate the severity of tissue inflammation. The activity of MPO in the colon tissue homogenate was determined by the kit method.
After the last administration for 24 h, the eyeballs were removed for blood collection, and the serum was obtained after centrifugation. The serum levels of IL-6, IL-10, IL-17, and TNF-α were measured with ELISA kits according to the manufacturer's instructions. All standards and samples were measured with a microplate reader at a wavelength of 450 nm.
The expression level of NF-κB p65 protein in colon tissue was detected by immunohistochemistry method and that of each layer of colon tissue was observed under microscope and recorded by photography. If the cytoplasm or nucleus of the cell have brown-yellow (brown) granules, it should be seen as positive cell for expressing NF-κB p65. The immunohistochemical sections of each group were randomly collected by GD-8 pathological image multimedia analysis system. At least three animal tissues were collected from each group, and ten high-power visual fields (20×) were selected to measure the area integral optical density (AIOD), which represented the staining intensity of the detected index, namely the expression quantity.
Protein was extracted as following: the tissues to be tested were cleaned by precooled PBS for three times, and added 1 ml RIPA lysate on the ice for lysing 20 min and then crushed with ultrasonic cell crusher in ice bath. After being centrifuged at 4℃, 12,500 rpm for 10 min, the supernatant was collected. The protein concentration of the supernatant was measured by BCA protein kit, and the concentration of protein in each group was calculated, then all the concentrations were adjusted to the lowest protein concentration with the RIPA lysate. Protein loading buffer was added and heated for 10 min at 100℃, sorting.
Proteins were resolved by 10% SDS-PAGE and transferred to PVDF membrane blocked with 5% milk for 30 min, and incubated overnight with antibodies at 1:1,000 dilution. Then the proteins were incubated with secondary antibodies at 1:5,000 for 60 min in thermostat. Signals were detected with an enhanced chemiluminescence kit and exposed in gel imager. The net optical density was quantifcationally analyzed with the gel imager analysis software (Peiqing, Shanghai, China).
All variables were expressed as the mean ± standard deviation. Statistical analysis was performed by using the SPSS 17.0.1 software package (SPSS Inc., Chicago, IL, USA). Sample mean differences between groups were analyzed by one-way ANOVA followed by a Tukey's post-hoc test. p < 0.05 was considered statistically significant.
On the 4th day of the experiment, DAI, including weight loss, stool occult blood and stool characteristics, was measured every two days until the end of the experiment. As shown in Fig. 1A–C, the indices of weight loss, stool occult blood and stool characteristics in the normal control group remained stable from 4th to 10th day, while the above indices in the model group were significantly increased. At the same time, the mice showed decreased activity, significant weight loss, poor hair smoothness, listlessness, soft stool or diarrhea, indicating that the UC model induced by 4% DSS was successful. Furthermore, the score of DAI was calculated by evaluating the weight loss, stool occult blood and stool characteristics. Consequently, the DAI of the model group was also significantly higher than that of the normal control group (Fig. 1D). On the other hand, these indices of each drug-treated group showed a downward trend, especially on the 10th day, the differences were statistically significant compared with the model group (p < 0.05 or p < 0.01). Meanwhile, the difference between the high-dose DLVT group and the model group on the 8th day was also statistically significant (p < 0.05 or p < 0.01). It is suggested that the clinical parameters and disease activity of UC induced by 4% DSS can be improved in each drug-treated group, but there was no significant difference between the drug groups.
Compared with the normal control group, the colon length in the model group decreased significantly. And each drug-treated group could significantly inhibit the shortening of colon length induced by DSS (p < 0.05 or p < 0.01) (Fig. 2A). Meanwhile, MPO activity of the colon tissue in the model group increased significantly, while that in each drug-treated group was significantly decreased compared with the model group (p < 0.05 or p < 0.01) (Fig. 2B). However, there was also no significant difference between the drug-treated groups.
Compared with the normal control group, the serum levels of IL-6, IL-17, and TNF-α in the model group increased significantly. After administration of each drug, the levels of the above inflammatory cytokines decreased significantly (p < 0.01). Among them, the decreased effect of high-dose DLVT on both IL-17 and TNF-α was significantly stronger than that of SASP (p < 0.01 or p < 0.05) (Fig. 3A–C). On the other hand, the serum level of IL-10 in the model group decreased significantly compared with the normal control group. And the IL-10 level in the drug-treated groups were all significantly increased compared with the model group (p < 0.01). In particular, the increased effect of high-dose DLVT on IL-10 was also stronger than that of SASP (p < 0.05) (Fig. 3D).
Firstly, in the normal control group, the colonic mucosa was intact and smooth with clear glandular layers. Almost no erosion, ulcer or inflammatory cell infiltration were observed. Secondly, in the model group, colonic mucosal epithelial erosion and glandular crypt destruction were observed under light microscope. Goblet cells were lost, with numerous inflammatory cell infiltrations such as neutrophils, lymphocytes, and plasma cells. Finally, in the DLVT, SASP, and LVT treated groups, the colonic mucosal damage was significantly reduced. The epithelial structure was intact, with ordered glandular crypts??arrangement; the inflammatory cells infiltration was decreased (Fig. 4A). The specimens of each group were observed under light microscope in ten high-power visual fields (20×), and histological injury score was obtained. As shown in Fig. 4B, the histological injury score of the model group was significantly increased compared with the normal control group. And the histological injury score of each drug-treated group was significantly lower than that of the model group (p < 0.05 or p < 0.01), indicating that the degree of pathological injury of colonic mucosa was significantly reduced.
Immunohistochemical staining of colon tissue showed that there was almost no expression of NF-κB p65 of colon tissue in the normal control group, and the number of positive cells expressing NF-κB p65 of colon tissue in the model group was significantly increased. Compared with the model group, the number of NF-κB p65 positive cells was significantly decreased in each drug-treated group (p < 0.01), but there was no significant difference between the drug-treated groups (Fig. 5A, B).
Electrophoresis scanning found that the NF-κB p65 expression in the model group was significantly higher than that of the normal group. Compared with the model group, the NF-κB p65 expression in each drug-treated group were all decreased significantly (p < 0.01), as shown in Fig. 6A, B.
Statins are one of the most widely used lipid-regulating drugs in clinical practice. Studies and case reports from large, randomized controlled trials showed that this kind of drugs have preventive and therapeutic effects on cardiovascular diseases such as atherosclerosis, thromboembolic diseases; immune inflammatory diseases such as UC, rheumatoid arthritis, ankylosing spondylitis and oxidative stress-related diseases [2526]. DLVT is a new member of the statins and is a dehydrogenation product of LVT. In this study, 4% DSS solution was used for free drinking to induce the establishment of a mouse UC model, and the improvement effect of two doses of DLVT on UC was studied by taking the drug of SASP, which is commonly used in the treatment of UC, as the control. The results showed that on the second day of free consumption of 4% DSS in mice, the body weight began to decline, stool characteristics changed, stool occult blood appeared and DAI increased. Meanwhile, the colon was significantly shortened and the mucosal lesions involved the distal colon in the UC mice. Microscopically, there was infiltration of inflammatory cells, multiple mucosal erosions or superficial ulcers, with cryptic abscess formation and vascular proliferation. On the other hand, the high dose of DLVT can significantly alleviate the clinical symptoms of UC and pathological damage of the colonic mucosa induced by DSS, and inhibit the shortening of colon length caused by inflammation. LVT and SASP also improved the above indices, but there was no significant difference compared with the high dose of DLVT.
It is generally believed that a variety of factors are involved in the pathogenesis of UC, and its chronic, recurrent clinical inflammatory manifestations involve chemotaxis, recruitment, infiltration, and release of inflammatory cytokines in many inflammatory cells [27]. Among them, the expression of NF-κB, the release of IL-6, IL-10, IL-17, and TNF-α, as well as the activity of MPO are important indices in evaluating the clinical symptoms and therapeutic effects of UC. NF-κB can regulate the expression of various inflammatory cytokines, such as IL-6, IL-10, IL-17, TNF-α, etc. [28]. The expression level of NF-κB in the colonic mucosa of UC patients is significantly increased, which plays a critical role in UC inflammation and is the target of many anti-inflammatory drugs [2930]. NF-κB is mainly composed of two subunits, p65 and p50. They combine to form a dimer, of which p65 is the main functional subunit with significant proinflammatory activity. Meanwhile, the increased levels of inflammatory cytokines such as IL-6, IL-17, and TNF-α are involved in the induction and formation of UC [31]. On the other hand, IL-10 is mainly synthesized and secreted by mononuclear macrophages and helper T cells. After binding to its own receptor, IL-10 can activate STAT3, thereby negatively regulating the transcription and secretion of inflammatory cytokines such as IL-1, IL-6, IL-8, and TNF-α [32]. Furthermore, MPO is an index of neutrophil recruitment in the UC model, and its activity specifically reflects the degree of colonic mucosal inflammation [3334]. Therefore, we investigated the effect of DLVT on the expression of these inflammatory factor levels and MPO activity. The results showed that besides the expression of NF-κB p65 in colonic mucosa, IL-6, IL-17, and TNF-α levels and MPO activity in UC mice were all significantly increased, while the level of IL-10 was significantly decreased. The above indices were significantly improved in each drug-treated group. In particular, the effect of both reducing IL-17, TNF-α and promoting IL-10 in the high-dose DLVT group was significantly stronger than that in the SASP group.
DLVT has a significant protective effect on UC induced by DSS in mice, which may be related to the inhibition of NF-κB p65 expression, the decrease of IL-6, IL-17, TNF-α levels and MPO activity, and the increase of IL-10 level. Considering that the effect of DLVT in both reducing IL-17, TNF-α and promoting IL-10 was significantly stronger than that of SASP, the molecular weight of DLVT is close to that of SASP (407.55/398.40), and the dose used of DLVT was eight times smaller than that of SASP, it is reasonable to assume that DLVT is superior to SASP on UC induced by DSS.
Figures and Tables
 | Fig. 1Changes of clinical parameters and disease activity in the mice.(A) Change of weight loss in the mice; (B) Change of stool occult blood in the mice; (C) Change of stool characteristics in the mice; (D) Change of Disease Activity Index (DAI) in the mice. Data are presented as mean ± standard deviation (n = 10 for each group). DLVT, dehydrolovastatin; LVT, lovastatin; SASP, salazosulfapyridine. *p < 0.05, **p < 0.01 compared with the model group.
|
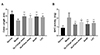 | Fig. 2Changes of colon length and myeloperoxidase (MPO) activity in the mice.(A) Change of colon length in the mice; (B) Change of MPO activity in the mice. Data are presented as mean ± standard deviation (n = 10 for each group). DLVT, dehydrolovastatin; LVT, lovastatin; SASP, salazosulfapyridine. *p < 0.05, **p < 0.01 compared with the model group.
|
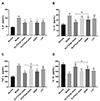 | Fig. 3Changes of serum levels of IL-6, IL-10, IL-17, and TNF-α in the mice.(A) Change of serum IL-6 level in the mice; (B) Change of serum IL-17 level in the mice; (C) Change of serum TNF-α level in the mice; (D) Change of serum IL-10 level in the mice. Data are presented as mean ± standard deviation (n = 10 for each group). DLVT, dehydrolovastatin; LVT, lovastatin; SASP, salazosulfapyridine. **p < 0.01 compared with the model group; #p < 0.05, ##p < 0.01 comparison between the high-dose DLVT group and SASP group.
|
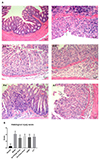 | Fig. 4Histological changes of colon tissue in the mice.(A) Histopathological HE staining of colon tissue at high-power visual fields (20×). Arrows mean exudation and inflammatory cells infiltration; (B) Histological injury score. Data are presented as mean ± standard deviation (n = 10 for each group). (A-a) Normal control group; (A-b) model group; (A-c) model group treated with high dose of DLVT (33.6 mg/kg); (A-d) model group treated with low dose of DLVT (16.8 mg/kg); (A-e) model group treated with SASP (250 mg/kg); (A-f) model group treated with LVT (16.8 mg/kg). DLVT, dehydrolovastatin; LVT, lovastatin; SASP, salazosulfapyridine. *p < 0.05, **p < 0.01 compared with the model group.
|
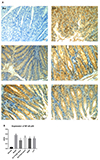 | Fig. 5Expression of NF-κB p65 protein of colon tissue in the mice.(A) Immunohistochemical staining of colon tissue at high-power visual fields (20×); (B) area integral optical density (AIOD). Data are presented as mean ± standard deviation (n = 10 for each group). (A-a) Normal control group; (A-b) model group; (A-c) model group treated with high dose of DLVT (33.6 mg/kg); (A-d) model group treated with low dose of DLVT (16.8 mg/kg); (A-e) model group treated with SASP (250 mg/kg); (A-f) model group treated with LVT (16.8 mg/kg). DLVT, dehydrolovastatin; LVT, lovastatin; SASP, salazosulfapyridine. **p < 0.01 compared with the model group.
|
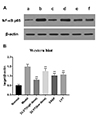 | Fig. 6Western blot.(A) Western blot results of NF-κB p65 protein expression; (B) Western blot results of NF-κB p65 protein levels were shown as the target/β-actin ratio of relative light unit values. Data are presented as mean ± standard deviation (n = 10 for each group). (a) Normal control group; (b) model group; (c) model group treated with high dose of DLVT (33.6 mg/kg); (d) model group treated with low dose of DLVT (16.8 mg/kg); (e) model group treated with SASP (250 mg/kg); (f) model group treated with LVT (16.8 mg/kg). DLVT, dehydrolovastatin; LVT, lovastatin; SASP, salazosulfapyridine. **p < 0.01 compared with the model group.
|
ACKNOWLEDGEMENTS
This study was supported by the Chongqing Health Bureau Project: Study on the therapeutic effect of dehydrolovastatin on inflammatory bowel disease. Project Number: 2013-2-152.
Notes
References
2. Amati L, Caradonna L, Leandro G, Magrone T, Minenna M, Faleo G, Pellegrino NM, Jirillo E, Caccavo D. Immune abnormalities and endotoxemia in patients with ulcerative colitis and in their first degree relatives: attempts at neutralizing endotoxin-mediated effects. Curr Pharm Des. 2003; 9:1937–1945.

3. Mitsuyama K, Toyonaga A, Sata M. Intestinal microflora as a therapeutic target in inflammatory bowel disease. J Gastroenterol. 2002; 37 Suppl 14:73–77.


4. Parlato M, Charbit-Henrion F, Hayes P, Tiberti A, Aloi M, Cucchiara S, Bègue B, Bras M, Pouliet A, Rakotobe S, Ruemmele F, Knaus UG, Cerf-Bensussan N. First identification of biallelic inherited DUOX2 inactivating mutations as a cause of very early onset inflammatory bowel disease. Gastroenterology. 2017; 153:609–611.e3.


5. Ng WK, Wong SH, Ng SC. Changing epidemiological trends of inflammatory bowel disease in Asia. Intest Res. 2016; 14:111–119.



6. Low END, Mokhtar NM, Wong Z, Raja Ali RA. Colonic mucosal transcriptomic changes in patients with long-duration ulcerative colitis revealed colitis-associated cancer pathways. J Crohns Colitis. 2019; 13:755–763.



7. Matsuoka K, Kobayashi T, Ueno F, Matsui T, Hirai F, Inoue N, Kato J, Kobayashi K, Kobayashi K, Koganei K, Kunisaki R, Motoya S, Nagahori M, Nakase H, Omata F, Saruta M, Watanabe T, Tanaka T, Kanai T, Noguchi Y, et al. Evidence-based clinical practice guidelines for inflammatory bowel disease. J Gastroenterol. 2018; 53:305–353.



8. Bernstein CN, Fried M, Krabshuis JH, Cohen H, Eliakim R, Fedail S, Gearry R, Goh KL, Hamid S, Khan AG, LeMair AW, Malfertheiner , Ouyang Q, Rey JF, Sood A, Steinwurz F, Thomsen OO, Thomson A, Watermeyer G. World Gastroenterology Organization Practice Guidelines for the diagnosis and management of IBD in 2010. Inflamm Bowel Dis. 2010; 16:112–124.


9. Piechota-Polanczyk A, Fichna J. Review article: the role of oxidative stress in pathogenesis and treatment of inflammatory bowel diseases. Naunyn Schmiedebergs Arch Pharmacol. 2014; 387:605–620.



10. Pedersen J, Coskun M, Soendergaard C, Salem M, Nielsen OH. Inflammatory pathways of importance for management of inflammatory bowel disease. World J Gastroenterol. 2014; 20:64–77.



11. Messaris E, Dassopoulos T. Concepts in inflammatory bowel disease management. In : Yeo CJ, editor. Shackelford's surgery of the alimentary tract. Philadelphia: Elsevier;2019. p. 1888–1918.
12. Seidelin JB, Coskun M, Nielsen OH. Mucosal healing in ulcerative colitis: pathophysiology and pharmacology. Adv Clin Chem. 2013; 59:101–123.

13. Pugliese D, Felice C, Papa A, Gasbarrini A, Rapaccini GL, Guidi L, Armuzzi A. Anti TNF-α therapy for ulcerative colitis: current status and prospects for the future. Expert Rev Clin Immunol. 2017; 13:223–233.

14. Ungaro R, Chang HL, Côté-Daigneault J, Mehandru S, Atreja A, Colombel JF. Statins associated with decreased risk of new onset inflammatory bowel disease. Am J Gastroenterol. 2016; 111:1416–1423.


15. Khalili H. Editorial: statins for inflammatory bowel disease: expanding the scope of prevention. Am J Gastroenterol. 2016; 111:1424–1426.


16. Côté-Daigneault J, Mehandru S, Ungaro R, Atreja A, Colombel JF. Potential immunomodulatory effects of statins in inflammatory bowel disease. Inflamm Bowel Dis. 2016; 22:724–732.


17. Li Y, Müller AL, Ngo MA, Sran K, Bellan D, Arora RC, Kirshenbaum LA, Freed DH. Statins impair survival of primary human mesenchymal progenitor cells via mevalonate depletion, NF-κB signaling, and Bnip3. J Cardiovasc Transl Res. 2015; 8:96–105.


18. Choi HW, Shin PG, Lee JH, Choi WS, Kang MJ, Kong WS, Oh MJ, Seo YB, Kim GD. Anti-inflammatory effect of lovastatin is mediated via the modulation of NF-κB and inhibition of HDAC1 and the PI3K/Akt/mTOR pathway in RAW264.7 macrophages. Int J Mol Med. 2018; 41:1103–1109.


19. Deng Q, Zhouq Q, Chen Y, Jin L, He Q. Relationship between lipidemia-modulating and anti-inflammatory effects of dehydrolovastatin. Chin J N Drugs. 2011; 20:162–166.
20. Deng Q, Yang Y, Zhou Q, Liu X, Gu Q. Experimental stdudy of dehydrolovastatin on adjuvant arthritis. Chongging Med J. 2015; 27:2320–2323.
21. Pandurangan AK, Ismail S, Saadatdoust Z, Esa NM. Allicin alleviates dextran sodium sulfate- (DSS-) induced ulcerative colitis in BALB/c mice. Oxid Med Cell Longev. 2015; 2015:605208.

22. Cooper HS, Murthy SN, Shah RS, Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993; 69:238–249.

23. Togre N, Bhoj P, Goswami K, Tarnekar A, Patil M, Shende M. Human filarial proteins attenuate chronic colitis in an experimental mouse model. Parasite Immunol. 2018; 40:e12511.

24. Zhang D, Ren YB, Wu HG, Yang YT, Wu LJ, Zhang J, Shi Z, Ma XP. Effect of different doses of herbal cake-partitioned moxibustion on histopathological changes of colon tissue in ulcerative colitis rats. Zhen Ci Yan Jiu. 2018; 43:68–74.

25. Agouridis AP, Elisaf MS, Nair DR, Mikhailidis DP. All for statins and statins for all; an update. Curr Pharm Des. 2016; 22:18–27.

26. Moeinian M, Abdolghaffari AH, Nikfar S, Momtaz S, Abdollahi M. Effects of alpha lipoic acid and its derivative "andrographolid-lipoic acid-1" on ulcerative colitis: a systematic review with meta-analysis of animal studies. J Cell Biochem. 2019; 120:4766–4782.


27. Sanchez-Munoz F, Dominguez-Lopez A, Yamamoto-Furusho JK. Role of cytokines in inflammatory bowel disease. World J Gastroenterol. 2008; 14:4280–4288.


28. Cortez M, Carmo LS, Rogero MM, Borelli P, Fock RA. A high-fat diet increases IL-1, IL-6, and TNF-α production by increasing NF-κB and attenuating PPAR-γ expression in bone marrow mesenchymal stem cells. Inflammation. 2013; 36:379–386.

29. Qian J, Zhao W, Miao X, Li L, Zhang D. Sam68 modulates apoptosis of intestinal epithelial cells via mediating NF-κB activation in ulcerative colitis. Mol Immunol. 2016; 75:48–59.


30. Hegazy SK, El-Bedewy MM. Effect of probiotics on pro-inflammatory cytokines and NF-kappaB activation in ulcerative colitis. World J Gastroenterol. 2010; 16:4145–4151.


31. Ito R, Kita M, Shin-Ya M, Kishida T, Urano A, Takada R, Sakagami J, Imanishi J, Iwakura Y, Okanoue T, Yoshikawa T, Kataoka K, Mazda O. Involvement of IL-17A in the pathogenesis of DSS-induced colitis in mice. Biochem Biophys Res Commun. 2008; 377:12–16.


32. Zhang D, Wei C, Yao J, Cai X, Wang L. Interleukin-10 gene-carrying bifidobacteria ameliorate murine ulcerative colitis by regulating regulatory T cell/T helper 17 cell pathway. Exp Biol Med (Maywood). 2015; 240:1622–1629.







 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download