Abstract
Objective
To compare patient survival outcomes between completion hysterectomy and conventional surveillance in locally advanced adenocarcinoma of the cervix after concurrent chemoradiotherapy (CCRT).
Methods
Patients with adenocarcinoma of the cervix after CCRT were identified in a tertiary academic center database from 2004 to 2018. Patients received completion hysterectomy or surveillance after CCRT. We compared the progression-free survival (PFS) and overall survival (OS) between the patients with or without adjuvant hysterectomy. Surgery features, operative complications, and pathologic characteristics were documented. Patient outcomes were also analyzed according to clinicopathologic factors.
Results
A total of 78 patients were assigned to completion surgery and 97 to surveillance after CCRT. The PFS was better in the surgery group compared to the CCRT only group, at 3 years the PFS rates were 68.1% and 45.2%, respectively (hazard ratio [HR]=0.46; 95% confidence interval [CI]=0.282–0.749; p=0.002). Adjuvant surgery was also associated with a higher rate of OS (HR=0.361; 95% CI=0.189–0.689; p=0.002), at 3 years, 87.9% and 67%, respectively. Tumor stage, size, lymph-vascular space invasion (LVSI), lymphadenopathy were associated with PFS but not with OS. Hysterectomy specimens revealed 64.1% (50/78) of the patients had pathologic residual tumor. Patients age less than 60, tumor size over 4 cm, stage IIB and persistent residual disease after CCRT were most likely to benefit from hysterectomy. Hysterectomy was associated with a lower rate of locoregional recurrence but did not reach statistical significance (5.13% vs. 13.5%, p=0.067).
References
1. Kokka F, Bryant A, Brockbank E, Powell M, Oram D. Hysterectomy with radiotherapy or chemotherapy or both for women with locally advanced cervical cancer. Cochrane Database Syst Rev. 2015. CD010260.

2. Albert A, Allbright R, Lee A, Vijayakumar S. Preoperative chemoradiation followed by hysterectomy for cervical cancer: patterns of care and survival in a large, hospital database. J Gynecol Oncol. 2019; 30:e41.

3. Wiebe E, Denny L, Thomas G. Cancer of the cervix uteri. Int J Gynaecol Obstet. 2012; 119(Suppl 2):S100–9.

4. Yang J, Shen K, Wang J, Yang J, Cao D. Extrafascial hysterectomy after concurrent chemoradiotherapy in locally advanced cervical adenocarcinoma. J Gynecol Oncol. 2016; 27:e40.

5. Platt SL, Patel A, Humphrey PJ, Al-Booz H, Bailey J. Completion surgery after chemoradiotherapy for cervical cancer – is there a role? UK Cancer Centre experience of hysterectomy post chemo-radiotherapy treatment for cervical cancer. J Obstet Gynaecol. 2019; 39:68–73.

6. Touboul C, Uzan C, Mauguen A, Gouy S, Rey A, Pautier P, et al. Prognostic factors and morbidities after completion surgery in patients undergoing initial chemoradiation therapy for locally advanced cervical cancer. Oncologist. 2010; 15:405–15.

7. Motton S, Houvenaeghel G, Delannes M, Querleu D, Soulé-Tholy M, Hoff J, et al. Results of surgery after concurrent chemoradiotherapy in advanced cervical cancer: comparison of extended hysterectomy and extrafascial hysterectomy. Int J Gynecol Cancer. 2010; 20:268–75.
8. Keys HM, Bundy BN, Stehman FB, Okagaki T, Gallup DG, Burnett AF, et al. Radiation therapy with and without extrafascial hysterectomy for bulky stage IB cervical carcinoma: a randomized trial of the Gynecologic Oncology Group. Gynecol Oncol. 2003; 89:343–53.

9. Morice P, Rouanet P, Rey A, Romestaing P, Houvenaeghel G, Boulanger JC, et al. Results of the GYNECO 02 study, an FNCLCC phase III trial comparing hysterectomy with no hysterectomy in patients with a (clinical and radiological) complete response after chemoradiation therapy for stage IB2 or II cervical cancer. Oncologist. 2012; 17:64–71.

10. Haque W, Verma V, Butler EB, Teh BS. Utilization of hysterectomy following chemoradiation for IB2/IIA2 cervical cancer in the National Cancer Data Base. Anticancer Res. 2018; 38:3175–9.
11. Favero G, Pierobon J, Genta ML, Araújo MP, Miglino G, Del Carmen Pilar Diz M, et al. Laparoscopic extrafascial hysterectomy (completion surgery) after primary chemoradiation in patients with locally advanced cervical cancer: technical aspects and operative outcomes. Int J Gynecol Cancer. 2014; 24:608–14.

Fig. 1.
PFS and OS between the 2 groups and subgroup analysis of patients with or without prRD. CCRT, concurrent chemoradiotherapy; CI, confidence interval; HR, hazard ratio; OS, overall survival; PFS, progression-free survival; psRD, post-radiation residual disease.
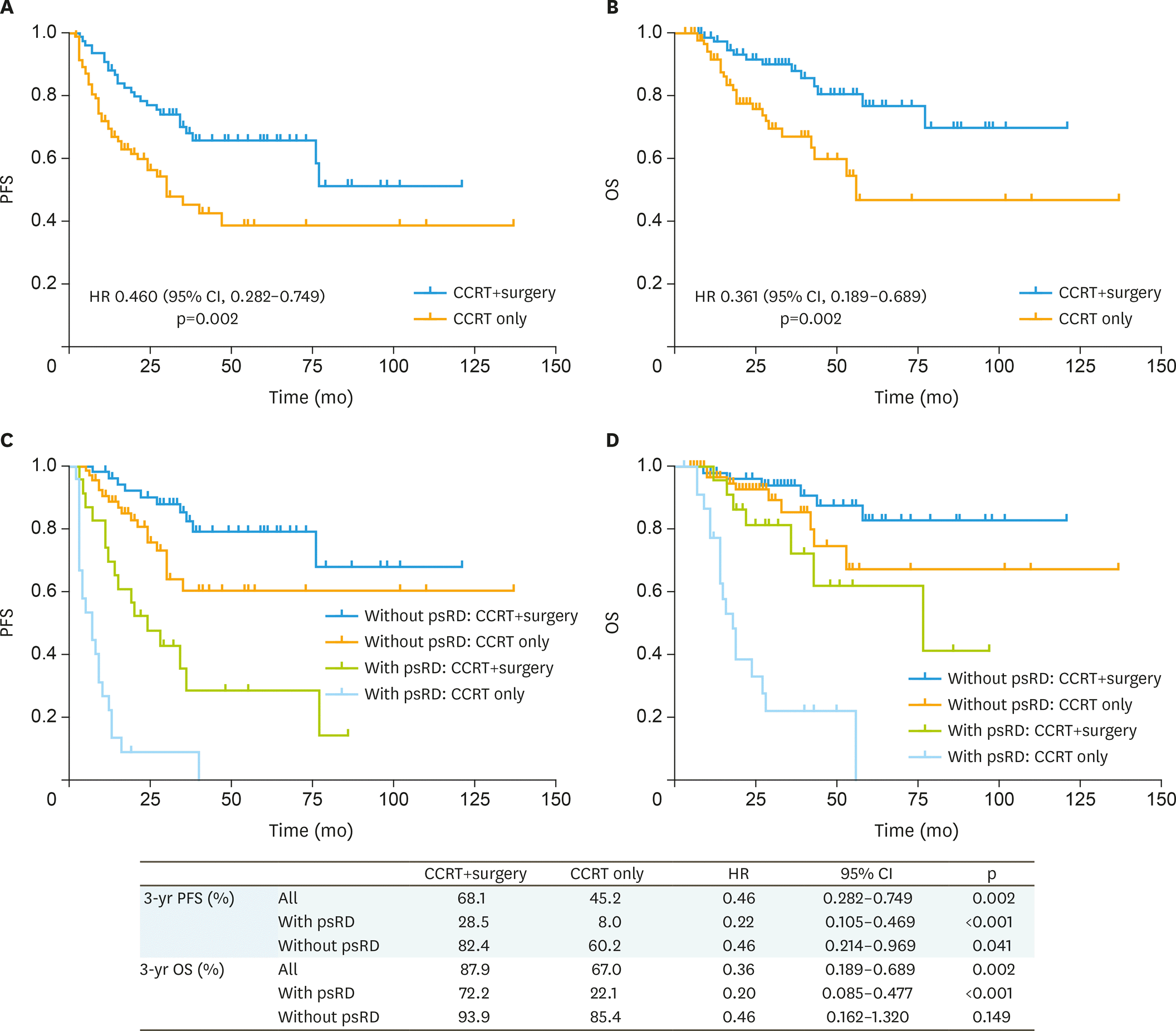
Fig. 2.
HR of completion surgery vs. surveillance for PFS and OS in specified clinical features. CCRT, concurrent chemoradiotherapy; CI, confidence interval; HR, hazard ratio; LAN, lymphoadenopathy; LVSI, lymph-vascular space invasion; OS, overall survival; PFS, progression-free survival; RD, residual disease.
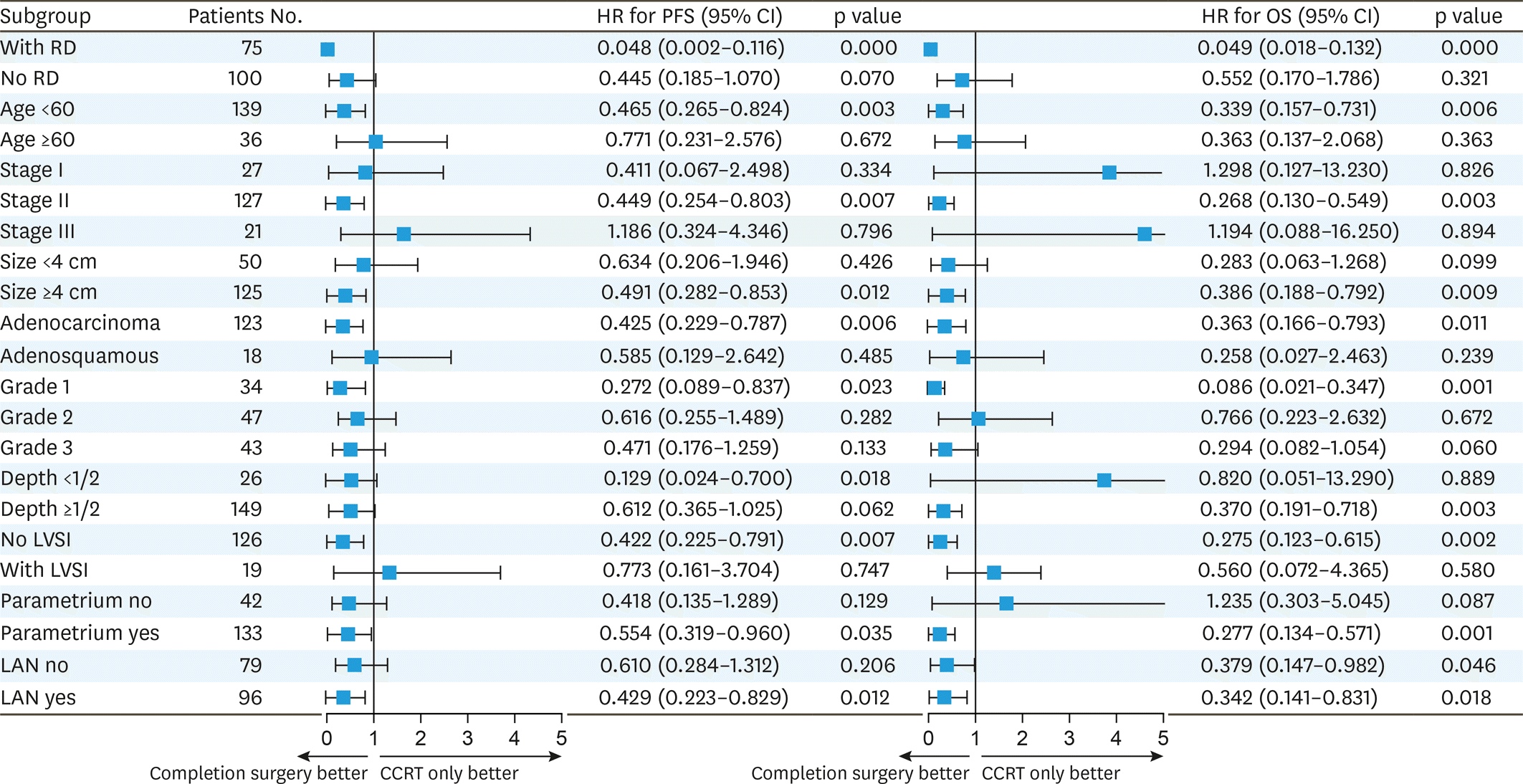
Table 1.
Patients' characteristics
| Characteristics | CCRT+hysterectomy (n=78) | CCRT only (n=97) | p value | |
|---|---|---|---|---|
| Age (yr) | 0.255 | |||
| Median (range) | 48 (22–77) | 54 (27–81) | ||
| <60 | 71 (91.0%) | 68 (70.1%) | 0.001 | |
| ≥60 | 7 (9.0%) | 29 (29.9) | ||
| Stage | 0.243 | |||
| IB | 16 (20.5%) | 12 (12.4%) | ||
| IIA | 5 (6.4%) | 8 (8.2%) | ||
| IIB | 51 (65.4%) | 62 (63.9%) | ||
| III | 6 (7.7%) | 15 (15.5%) | ||
| Size (cm) | 0.810 | |||
| <2 | 3 (3.8%) | 4 (4.1%) | ||
| 2–4 | 21 (26.9%) | 22 (22.7%) | ||
| ≥4 | 54 (69.2%) | 71 (73.2%) | ||
| Histologic type | 0.084 | |||
| Adeno- | 53 (67.9%) | 70 (72.2%) | ||
| Adenosquamous | 5 (6.4%) | 13 (13.4%) | ||
| Mixed adeno-type* | 20 (25.6%) | 14 (14.4%) | ||
| Grade | 0.147 | |||
| Well | 20 (25.6%) | 17 (17.5%) | ||
| Moderate | 25 (32.1%) | 22 (22.7%) | ||
| Poor | 15 (19.2%) | 28 (28.9%) | ||
| Unknown | 18 (23.1%) | 30 (30.9%) | ||
| Lymphadenopathy | 0.948 | |||
| Yes | 35 (44.9%) | 44 (45.4%) | ||
| No | 43 (55.1%) | 53 (54.6%) | ||
| CA-125 abnormal | 32/68 (47.1%) | 39/75 (52%) | 0.672 | |
| SCC-Ag abnormal | 12/60 (20%) | 22/69 (31.9%) | 0.184 | |
| NACT | 45/78 (57.7%) | 22/97 (22.7%) | <0.005 | |
| Stromal invasion | Pre-treatment† | Pathological‡ | Pre-treatment† | 0.393 |
| <1/2 | 14 (17.9%) | 25 (32.1%) | 12 (12.4%) | |
| ≥1/2 | 64 (82.1%) | 25 (32.1%) | 85 (87.6%) | |
| Parametrium invasion | Pre-treatment† | Pathological‡ | Pre-treatment† | 0.287 |
| Yes | 56 (71.8%) | 6 (7.7%) | 77 (79.4%) | |
| No | 22 (28.2%) | 44 (56.4%) | 20 (20.6%) | |
| LVSI | Pre-treatment† | Pathological‡ | Pre-treatment† | 0.416 |
| Negative | 54 (69.2%) | 58 (74.4%) | 69 (71.1%) | |
| Positive | 7 (8.9%) | 10 (12.8%) | 9 (9.3%) | |
| Unknown | 17 (21.8%) | 10 (12.8%) | 19 (19.6%) | |
| RD | Post-radiation§ | Pathological‡ | Post-radiation§ | 0.706 |
| Yes | 23 (29.5%) | 50 (64.1%) | 25 (25.8%) | |
| No | 55 (70.5%) | 28 (35.9%) | 72 (74.2%) | |
CA-125, cancer antigen 125; CCRT, concurrent chemoradiotherapy; LVSI, lymph-vascular space invasion; NACT, neoadjuvant chemotherapy; RD, residual disease; SCC-Ag, squamous cell carcinoma antigen.
* Mixed adenocarcinoma includes minimal deviation adenocarcinoma, adenocarcinoma with villoglandular differentiation, adenocarcinoma with mucinous differentiation, adenocarcinoma with clear cell differentiation;
Table 2.
The univariate and multivariate analysis of clinicopathological factors and survival outcomes
Table 3.
Factors related to RD




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download