Abstract
PURPOSE
The aim of this study was to investigate the effect of different numbers of heat treatments applied to superstructure porcelain on optical, thermal, and phase formation properties of zirconia.
MATERIALS AND METHODS
Forty zirconia specimens were prepared in the form of rectangular prism. Specimens were divided into four groups (n = 10) according to the number of firing at heating values of porcelain. Color differences and translucency parameter were measured, and X-ray diffraction (XRD) analysis and differential scanning calorimetry (DSC) were performed. Data were analyzed with analysis of variance (ANOVA).
Zirconia material, which has superior mechanical properties, was first used in the late 1960s1 as a biomaterial. It was introduced into the field of dentistry in the 1990s and is now used frequently in many applications at dental clinics.12 Zirconia is widely used in clinical applications of prosthetic dentistry as a framework material for all-ceramic restorations.3 Its good mechanical properties, biocompatibility, and structural resistance to tensile strength allow for its use in both anterior and posterior region single- and multi-unit restorations.4 Restorations with zirconia frameworks can be produced with computer-aided design (CAD) and computer-aided manufacturing (CAM) systems.5 Such restorations involve applying the veneer with traditional feldspathic porcelain to achieve a more natural look.6
Zirconia, which is a polymorphic material containing cubic, monoclinic, and tetragonal phases in its structure, remains in the tetragonal phase at room temperature. It also includes yttrium oxide, doped at the rate of 2 – 3%, in its structure.1 Through a mechanism called transformation hardening, compression pressure is applied by absorbing the crack energy of the tetragonal phase, increasing the particle size, and preventing spreading of the crack.789 However, in the presence of moisture, monoclinic phase conversion (t→m) can be triggered by different stimuli, such as stress concentration leading to low temperature degradation or low temperature aging.10 Studies that have examined the tetragonal-monoclinic (t-m) phase change show that the heat treatment application reverses the monoclinic phase to the tetragonal phase and eliminates the residual stress11 but does not have the potential to reduce any defects on the yttrium tetragonal zirconia polycrystalline (Y-TZP) ceramics.12
Esthetics is one of the leading factors for all-ceramic restoration. The restoration and the natural teeth must be similar, and this similarity is affected by many factors, such as translucency, fluorescence, surface properties, and shape.13 Naturally, it is very difficult for a restoration to be appreciated at once by the patient. It may be necessary to resend the work to the laboratory a number of times for various corrections. The restoration for correction will be fired again and exposed to high temperatures. Previous studies141516 have shown that the color of esthetic materials does not change with repetitive firing. However, O'Brien et al.17 found the color changes between specimens fired 3 times and those done 6 times (ΔE=1). They also stated that changes in sintering temperatures and amounts of time can affect the zirconia particle sizes, microstructures, and possibly their optical properties.181920 The color properties of natural teeth and restorative materials in dentistry can be measured using the Commission Internationale de l'Eclairage (CIE) color system.21 The ΔE values in these measurements are evaluated as ΔE < 3, not clinically detectable, ΔE = 3 – 5, clinically acceptable, and ΔE > 5, clinically unacceptable.22 Optical properties such as color stability and translucency parameters (TP) are thought to affect the esthetic properties of materials.23 The TP is determined by calculating the color difference after measuring the color of the material with uniform thickness in black and white backgrounds.24
Differential scanning calorimetry (DSC), thermal gravimetric (TG), and x-ray diffraction analysis (XRD) methods are used to examine the changes in the thermal properties and phase formation of polymorphic materials such as zirconia. Thermal analysis is a complementary technique that measures the enthalpy of the system with the temperature-dependent weight changes (TG) of the materials and the heat capacity (DSC) of the reactions when the temperatures increase.25 XRD analysis is a method for examining the characterization and phase transformations of a material.26
The aim of this study is to investigate the effects of repetitive firing processes on the optical, thermal, and phase changes of the zirconia framework. One of the hypotheses of the study is that the increase in the number of firings of the material will lead to a change in its color and translucency. Our second hypothesis is that the additional firing processes will lead to phase changes of the material.
The study included 40 rectangular prism zirconia framework specimens. These were produced by scraping with the help of the CAD/CAM unit (Yenadent D40 CAM unit; Yenadent, ZenoTec, Istanbul, Turkey), in a 12 × 4 × 2 mm thickness from A1 color Prettau blocks (Zirkonzahn GmbH, Gais, Italy). The sintering process of the specimens was carried out in the sintering furnace (Zirkonofen, Gais, Italy) according to the manufacturer's instructions, with an initial temperature of 300℃, a waiting time of 30 minutes, and a final temperature that reached 1,540℃ in 60 minutes and remained at 1,540℃ for 90 minutes. The specimens obtained were divided into four groups according to the firing processes (n = 10):
Group 1: Heat treatments were applied to the zirconia framework specimens without any superstructure porcelain. The initial temperature in these processes was 600℃, the waiting time was 7 minutes, the heat increase was 65℃ per minute, the final temperature was 925℃, and the waiting time was 12 minutes. These specimens were then subjected to the heat treatment applied in the glazing process. The initial temperature in these processes was 600℃, the drying time was 7 minutes, the final temperature was 895℃, and the waiting time was 12 minutes. The processes were carried out according to the recommendations of the manufacturer. These specimens formed the control group.
Group 2: In addition to the heat treatments applied in Group 1, the same heat treatments were repeated 1 more time.
Group 3: In addition to the heat treatments applied in Group 1, the same heat treatments were repeated 2 more times.
Group 4: In addition to the heat treatments applied in Group 1, the same heat treatments were repeated 3 more times.
As a result of the firing processes, specimens were obtained from those that had been fired just once and those fired 1, 2 and 3 more times. The firing process of the specimens was carried out by the same technician in the laboratory.
The fired specimens were kept in distilled water at 4℃ until color measurements were made. The color and TP measurements were verified with a digital caliper that confirmed the thickness of the specimens as 2 mm, and the measurement process was started. The color measurements of the specimens were made by a single researcher with the help of a spectrophotometer (VITA EasyShade V; VITA Zahnfabrik, Bad Sackingen, Germany) and with a grey-tone background.27 All the measurements of the specimens placed on the background were performed in the single tooth mode of the spectrophotometer. The mean value was obtained from the right, left, and middle regions of each specimen by taking three measurements on the grey, white, and black backgrounds. Before each measurement, the spectrophotometer was calibrated according to the recommendations of the manufacturer. The color values of all specimens were recorded according to the CIE L*a*b* system. With the formula ΔE* = [(ΔL*)2 + (Δa*)2 + (Δb*2]1/2, the mean values and the differences were obtained for each specimen.28 The TP was calculated by placing the Lw, aw, bw and Lb, ab, bb values (as obtained by the spectrophotometer) of the specimens placed on the white (w) and black (b) backgrounds into the following formula: TP = ([Lb − Lw]2 + [ab − aw]2 + [bb − bw]2)1/2.29
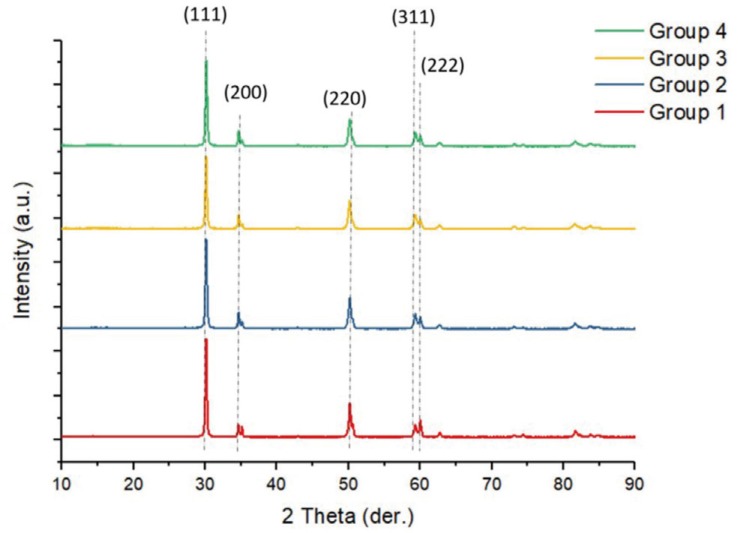
XRD analysis was performed to evaluate the effect of the repetitive firing processes on the phase analysis of the zirconia specimens. One randomly selected specimen from each group was tested in the XRD device (EMPYREAN, PANalytical, Lelyweg, The Netherlands) using the parameters of a wavelength of 1.5419 (Kα), a scan range from 10° – 90°, a step size of 0.0263°, and a scan speed of 0.067°/s. The graphs of the XRD analysis data were obtained using the Origin Pro 8.1 software (OriginLab Corporation, Northampton, MA, USA).
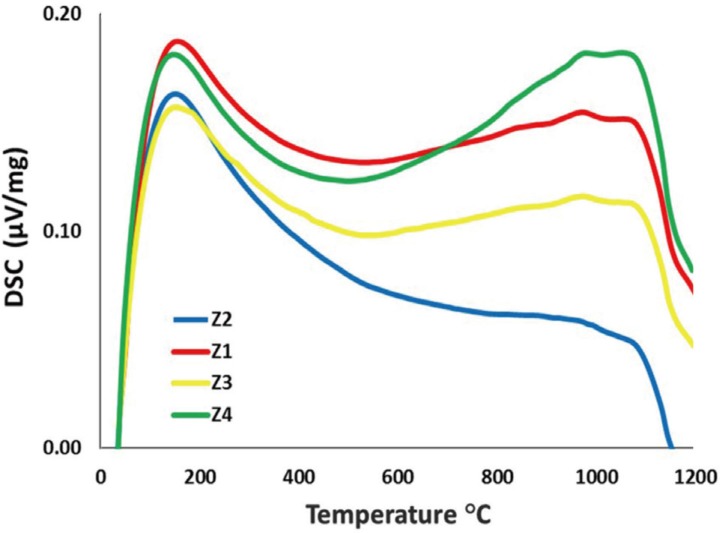
To examine the thermal changes of the zirconia specimens resulting from the repetitive firing processes, the specimens placed in the Differential Scanning Calorimeter (NETZSCH STA 409 PC Luxx, Bavaira, Germany) were heated at a temperature rate of 10℃/min at 0 – 1,200℃. Each specimen was randomly selected from the groups and placed in a platinum specimen holder. The weight and structural changes of the zirconia specimens against the temperatures were then examined.
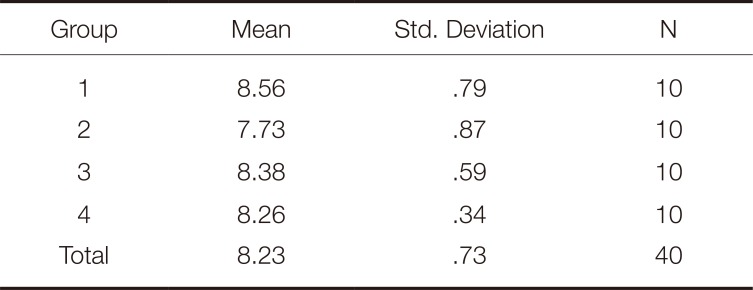
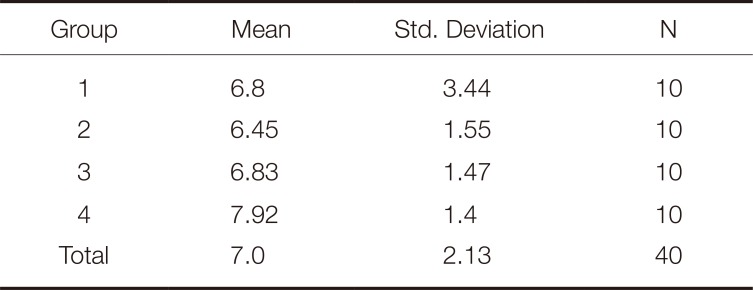
As a result of the analysis of variance (ANOVA), it was observed that there was no statistically significant difference in the ΔE, TP, L, a, and b value changes of the zirconia specimens as a result of the repetitive firing processes (P > .05). The mean ΔE and TP values of the repetitive firing processes are shown in Table 1 and Table 2.
The XRD results of the zirconia specimens are presented in Figure 1. In all the specimens, the main phase is zirconium oxide. The major peaks of zirconium oxide are found at 2θ values of 30° (111) and 50° (220). Zirconium oxide peaks reached almost the same height in all groups. Only the peak density of the tetragonal (220) phase increased along with the number of heat treatments.
The DSC results of the zirconia specimens are presented in Figure 2. After heating the specimens from room temperature to 1,200℃, a 180℃ exothermic peak occurred in the zirconia specimens of all the groups. Furthermore, the exothermic peak was observed at 1,100℃. This is because the tetragonal ZrO2 phase increased.30
The aim of this study is to investigate the effect of repetitive firing processes on the optical, thermal, and phase formation changes of the zirconia framework. According to the results, because the color and translucency of zirconia did not exhibit statistically significant differences after the additional firing processes, our initial hypothesis was rejected. In the XRD analysis, there were no differences in the phases and peak levels of the materials belonging to the groups, whereas in the DSC analysis, differences were observed in the peak levels of the groups. As a result of the additional firing processes, because zirconia specimens exhibited thermal differences but no phase differences, our second hypothesis was also rejected.
Color is one of the most important parameters affecting the esthetic and clinical success of a restoration. The optical property of the framework material is an important factor in the final shade of the restoration.31 There may be changes in the optical properties of restorations due to the sintering process, the application techniques for veneer ceramics, the firing temperatures, the glazing processes, and the laboratory procedures.32 The literature includes studies reporting that heating processes with high temperatures affect the optical properties of zirconia materials.3334 One study found that the sintering processes at high temperatures for long periods caused a decrease in the ΔE values of the zirconia specimens and affected the final colors.19 In their study, Öztürk et al.35 observed significant changes in L, a, and b values as a result of increasing the firing of DC-Zircon crowns with zirconia frameworks from 3 to 9 times. Heffernan et al.13 also reported that additional firing processes resulted in significant changes in the translucency values of the all-ceramic systems. Bachhav and Aras36 found that firing processes repeated 1, 3, 5, and 9 times affected the L, a, and b values of the all-ceramics, while Li et al.37 reported that firing processes repeated 1, 3, and 5 times affected the TP and ΔE values of the all-ceramics. Fathi et al.38 found that repeated firings and different porcelain veneer thicknesses affected the final colors and translucencies of the zirconia systems. Zirconia ceramics have a white color and are classified as semi-translucent materials.39 It is known that zirconia frameworks exhibit less translucency than monolithic zirconia.40 The mean TP value was reported to be 16.4 for monolithic zirconium with a thickness of 1 mm and 7.0 for the zirconia framework with the same thickness.41 In the present study, a 2 mm thick zirconia framework was used and the mean ΔE and TP values were found to be 8.23 and 7.0. While the TP values in this study are similar to those previously mentioned in the literature, the ΔE values are well above the clinically acceptable values indicated by O'Brien et al..17 We believe that this difference is caused by the high thickness of the materials used in the study and the rather dense structure of the zirconia. This finding also indicates that zirconia frameworks should be supported with superstructure porcelain. At the same time, the results of our study showed that repetitive firing processes did not affect the colors and translucencies of the zirconia frameworks. Although this result differs from the data in the literature, we believe that the difference is caused by the limitation of the firing processes to 4 times or the thickness of the specimens being 2 mm.
Zirconia framework restorations are subjected to heat treatment during the application of the superstructure porcelain. It was reported that there may be changes in the tetragonal-monoclinic phase transformations and mechanical properties of the material due to these temperature changes.42 The effects of the heat increases and the heat applications on the microstructure of zirconia have been emphasized in recent studies. Passos et al.43 reported that the application of heat treatment following sandblasting on zirconia specimens caused tetragonal-monoclinic phase transformations.
Vatali et al.42 reported that the combined applications of heat and aging treatments resulted in the tetragonal-monoclinic transformation of zirconia. Song et al.44 also reported complete m-t phase transformation in zirconia specimens that were exposed to temperatures of 500 – 1,000℃ for 15 minutes. However, Sato et al.45 reported that the monoclinic phase content of the zirconia that was subjected to heat treatment at 500 – 1,200℃ for 5 minutes remained on the surface, and Ebeid et al.19 reported that the temperature and time changes in the sintering parameters did not cause tetragonal-monoclinic phase transformations. Alkurt et al.46 reported that additional firing processes carried out 2, 5, and 10 times did not cause tetragonal-monoclinic phase transformation in zirconia specimens. Also, in their study on monolithic zirconia, Öztürk and Çelik,47 reported that the highest peak was in the tetragonal phase, and there was no tetragonal-monoclinic transformation. According to the XRD results of our study, the main phase was zirconium oxide in all specimens, no tetragonal-monoclinic phase transformation was observed due to repetitive firing processes, and the peak density of the tetragonal phase increased due to heat treatments. We believe that these differences in the literature are due to the types of zirconia used and the heating temperatures.
DSC is a thermal technique used to examine physical and chemical changes, endothermic and exothermic processes due to temperatures, and heat transitions in the material.48 This technique also helps examine the changes in polymorphic materials such as zirconia against temperature changes and the phase transformations. In the thermal analysis, the highest peak density was observed in the specimens fired 1 time in temperature changes increasing up to 200℃, while the specimens fired 2 and 3 times showed the lowest peak densities. Depending on the temperature increases, the peak densities of the specimens fired 2 times decreased and these specimens also showed the lowest peak densities. At higher temperatures (1,000 – 1,200℃), the specimens fired 4 times reached the highest peak densities. It is thought that the reason for this is that the specimens fired 4 times were exposed to higher temperatures, and this caused an increase in the tetragonal phase.
The XRD and DSC analyses showed that the specimens belonging to all groups remained in the tetragonal phase, no changes were observed while the main structures of the specimens were preserved, and no tetragonal-monoclinic phase transformation occurred due to the repeated heat treatments. It is indicated by the results of this study that a material such as zirconia that is exposed to very high temperatures retains its structural and optical properties in thermal changes. One of the limitations of this study is that the zirconia specimens used have a single thickness, and the values of specimens with different thicknesses were not therefore compared. Another limitation is that color measurements were made only for the frameworks, regardless of the superstructure porcelain.
The following conclusions were reached within the scope of this study. Firing processes performed 1, 2, 3, and 4 times did not affect the color and translucency changes of the zirconia frameworks. In addition, repetitive firing processes did not cause any changes in the phase formation of zirconia frameworks. Nevertheless, in the thermal analysis, the highest peak densities due to temperature increases were obtained in the specimens that were fired 4 times, and the lowest peak densities were obtained in the specimens that were fired 2 times.
ACKNOWLEDGEMENTS
The authors are grateful to Memiş Özdemir for his assistance with statistical analysis and Hatice Bayrakçeken for her assistance with thermal analysis.
References
1. Piconi C, Maccauro G. Zirconia as a ceramic biomaterial. Biomaterials. 1999; 20:1–25. PMID: 9916767.

2. Clarke IC, Manaka M, Green DD, Williams P, Pezzotti G, Kim YH, Ries M, Sugano N, Sedel L, Delauney C, Nissan BB, Donaldson T, Gustafson GA. Current status of zirconia used in total hip implants. J Bone Joint Surg Am. 2003; 85-A:73–84.

3. Ban S. Properties of zirconia for realization of all-ceramic restoration. J Tokyo Dent Coll Soc. 2007; 107:670–684.
4. Koçak EF, Uçar Y, Kurtoğlu C, Johnston WM. Color and translucency of zirconia infrastructures and porcelain-layered systems. J Prosthet Dent. 2019; 121:510–516. PMID: 30477923.

5. Miyazaki T, Hotta Y, Kunii J, Kuriyama S, Tamaki Y. A review of dental CAD/CAM: current status and future perspectives from 20 years of experience. Dent Mater J. 2009; 28:44–56. PMID: 19280967.

6. Prasad HA, Pasha N, Hilal M, Amarnath GS, Kundapur V, Anand M, Singh S. To evaluate effect of airborne particle abrasion using different abrasives particles and compare two commercial available zirconia on flexural strength on heat treatment. Int J Biomed Sci. 2017; 13:93–112. PMID: 28824346.
7. Luthardt RG, Holzhüter M, Sandkuhl O, Herold V, Schnapp JD, Kuhlisch E, Walter M. Reliability and properties of ground Y-TZP-zirconia ceramics. J Dent Res. 2002; 81:487–491. PMID: 12161462.
8. Chevalier J. What future for zirconia as a biomaterial? Biomaterials. 2006; 27:535–543. PMID: 16143387.

9. Kelly JR, Denry I. Stabilized zirconia as a structural ceramic: an overview. Dent Mater. 2008; 24:289–298. PMID: 17624420.

10. Souza RO, Valandro LF, Melo RM, Machado JP, Bottino MA, Ozcan M. Air-particle abrasion on zirconia ceramic using different protocols: effects on biaxial flexural strength after cyclic loading, phase transformation and surface topography. J Mech Behav Biomed Mater. 2013; 26:155–163. PMID: 23746698.

11. Ramos GF, Pereira GK, Amaral M, Valandro LF, Bottino MA. Effect of grinding and heat treatment on the mechanical behavior of zirconia ceramic. Braz Oral Res. 2016; 30:S1806-83242016000100012.

12. Aurélio IL, Dorneles LS, May LG. Extended glaze firing on ceramics for hard machining: Crack healing, residual stresses, optical and microstructural aspects. Dent Mater. 2017; 33:226–240. PMID: 28069245.

13. Heffernan MJ, Aquilino SA, Diaz-Arnold AM, Haselton DR, Stanford CM, Vargas MA. Relative translucency of six all-ceramic systems. Part II: core and veneer materials. J Prosthet Dent. 2002; 88:10–15. PMID: 12239473.

14. Barghi N, Goldberg . Porcelain shade stability after repeated firing. J Prosthet Dent. 1977; 37:173–175. PMID: 264553.

15. Jorgenson MW, Goodkind RJ. Spectrophotometric study of five porcelain shades relative to the dimensions of color, porcelain thickness, and repeated firings. J Prosthet Dent. 1979; 42:96–105. PMID: 287794.

16. Barghi N, Lorenzana RE. Optimum thickness of opaque and body porcelain. J Prosthet Dent. 1982; 48:429–431. PMID: 6957597.
17. O'Brien WJ, Kay KS, Boenke KM, Groh CL. Sources of color variation on firing porcelain. Dent Mater. 1991; 7:170–173. PMID: 1813339.
18. Matsui K, Yoshida H, Ikuhara Y. Isothermal sintering effects on phase separation and grain growth in yttria-stabilized tetragonal zirconia polycrystal. J Am Ceram Soc. 2009; 92:467–475.

19. Ebeid K, Wille S, Hamdy A, Salah T, El-Etreby A, Kern M. Effect of changes in sintering parameters on monolithic translucent zirconia. Dent Mater. 2014; 30:e419–e424. PMID: 25262211.

20. Stawarczyk B, Emslander A, Roos M, Sener B, Noack F, Keul C. Zirconia ceramics, their contrast ratio and grain size depending on sintering parameters. Dent Mater J. 2014; 33:591–598. PMID: 24998170.

21. Nogueira AD, Della Bona A. The effect of a coupling medium on color and translucency of CAD-CAM ceramics. J Dent. 2013; 41:e18–e23. PMID: 23438417.

22. Alghazali N, Burnside G, Moallem M, Smith P, Preston A, Jarad FD. Assessment of perceptibility and acceptability of color difference of denture teeth. J Dent. 2012; 40:e10–e17. PMID: 22561647.

23. Shiraishi T, Watanabe I. Thickness dependence of light transmittance, translucency and opalescence of a ceria-stabilized zirconia/alumina nanocomposite for dental applications. Dent Mater. 2016; 32:660–667. PMID: 26925845.

24. Ikeda T, Sidhu SK, Omata Y, Fujita M, Sano H. Colour and translucency of opaque-shades and body-shades of resin composites. Eur J Oral Sci. 2005; 113:170–173. PMID: 15819825.

25. Silva LH, Costa AK, Queiroz JR, Bottino MA, Valandro LF. Ceramic primer heat-treatment effect on resin cement/Y-TZP bond strength. Oper Dent. 2012; 37:634–640. PMID: 22621166.

26. Srinivasan R, Davis HB, Cavin OB, Hubbard CR. Crystallization and phase transformation process in zirconia: An in situ high-temperature x-ray diffraction study. J Am Ceram Soc. 1992; 75:1217–1222.

27. Shokry TE, Shen C, Elhosary MM, Elkhodary AM. Effect of core and veneer thicknesses on the color parameters of two all-ceramic systems. J Prosthet Dent. 2006; 95:124–129. PMID: 16473086.

28. Knispel G. Factors affecting the process of color matching restorative materials to natural teeth. Quintessence Int. 1991; 22:525–531. PMID: 1882045.
29. Turgut S, Bagis B, Turkaslan SS, Bagis YH. Effect of ultraviolet aging on translucency of resin-cemented ceramic veneers: an in vitro study. J Prosthodont. 2014; 23:39–44. PMID: 23725214.

30. Isfahani TD, Javadpour J, Khavandi A, Goodarzi M, Rezaie HR. Nanocrystalline growth activation energy of zirconia polymorphs synthesized by mechanochemical technique. J Mater Sci Technol. 2014; 30:387–393.
31. Abualsaud H, Zandparsa R, Hirayama H, Sadig W, Aboushelib M, Salameh Z. Color management of the cervical region using different framework materials. J Esthet Restor Dent. 2011; 23:371–378. PMID: 22142295.

32. Tabatabaian F. Color in zirconia-based restorations and related factors: A literature review. J Prosthodont. 2018; 27:201–211. PMID: 29315947.

33. Lawson NC, Maharishi A. Strength and translucency of zirconia after high-speed sintering. J Esthet Restor Dent. 2019; 9. 13.

34. Juntavee N, Attashu S. Effect of sintering process on color parameters of nano-sized yttria partially stabilized tetragonal monolithic zirconia. J Clin Exp Dent. 2018; 10:e794–e804. PMID: 30305879.

35. Ozturk O, Uludag B, Usumez A, Sahin V, Celik G. The effect of ceramic thickness and number of firings on the color of two all-ceramic systems. J Prosthet Dent. 2008; 100:99–106. PMID: 18672126.

36. Bachhav VC, Aras MA. The effect of ceramic thickness and number of firings on the color of a zirconium oxide based all ceramic system fabricated using CAD/CAM technology. J Adv Prosthodont. 2011; 3:57–62. PMID: 21814612.

37. Li S, Pang L, Yao J. The effects of firing numbers on the opening total pore volume, translucency parameter and color of dental all-ceramic systems. Hua Xi Kou Qiang Yi Xue Za Zhi. 2012; 30:417–419. PMID: 22934503.
38. Fathi A, Farzin M, Giti R, Kalantari MH. Effects of number of firings and veneer thickness on the color and translucency of 2 different zirconia-based ceramic systems. J Prosthet Dent. 2019; 122:565. PMID: 31699449.

39. Vichi A, Louca C, Corciolani G, Ferrari M. Color related to ceramic and zirconia restorations: a review. Dent Mater. 2011; 27:97–108. PMID: 21122905.

40. Tuncel İ, Turp I, Üşümez A. Evaluation of translucency of monolithic zirconia and framework zirconia materials. J Adv Prosthodont. 2016; 8:181–186. PMID: 27350851.

41. Fathy SM, El-Fallal AA, El-Negoly SA, El Bedawy AB. Translucency of monolithic and core zirconia after hydrothermal aging. Acta Biomater Odontol Scand. 2015; 1:86–92. PMID: 27335897.

42. Vatali A, Kontonasaki E, Kavouras P, Kantiranis N, Papadopoulou L, Paraskevopoulos KK, Koidis P. Effect of heat treatment and in vitro aging on the microstructure and mechanical properties of cold isostatic-pressed zirconia ceramics for dental restorations. Dent Mater. 2014; 30:e272–e282. PMID: 24950805.

43. Passos SP, Linke B, Major PW, Nychka JA. The effect of air-abrasion and heat treatment on the fracture behavior of Y-TZP. Dent Mater. 2015; 31:1011–1021. PMID: 26117560.

44. Song JY, Park SW, Lee K, Yun KD, Lim HP. Fracture strength and microstructure of Y-TZP zirconia after different surface treatments. J Prosthet Dent. 2013; 110:274–280. PMID: 24079562.

45. Sato H, Yamada K, Pezzotti G, Nawa M, Ban S. Mechanical properties of dental zirconia ceramics changed with sandblasting and heat treatment. Dent Mater J. 2008; 27:408–414. PMID: 18717169.

46. Alkurt M, Yeşil Duymus Z, Gundogdu M. Effects of multiple firings on the microstructure of zirconia and veneering ceramics. Dent Mater J. 2016; 35:776–781. PMID: 27725514.

47. Öztürk C, Çelik E. Influence of heating rate on the flexural strength of monolithic zirconia. J Adv Prosthodont. 2019; 11:202–208. PMID: 31497267.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download