Abstract
PURPOSE
To investigate the influence of crown material (lithium-disilicate, 3Y-TZP zirconia) and abutment type (rigid implant, resin tooth with artificial periodontium) on wear performance of their antagonist teeth and adjacent teeth.
MATERIALS AND METHODS
A mandibular left first molar (#36) with adjacent human teeth (mandibular left second premolar: #35, mandibular left second molar: #37) and antagonistic human teeth (maxillary left second premolar: #25, maxillary left first molar: #26, maxillary left second molar: #27) was prepared simulating a section of the jaw. Samples were made with extracted human molars (Reference), crowned implants (Implant), or crowned resin tooth analogues (Tooth). Crowns (tooth #36; n = 16/material) were milled from lithium-disilicate (Li, IPS e.max CAD) or 3Y-TZP zirconia (Zr, IPS e.max ZirCAD, both Ivoclar Vivadent). Thermal cycling and mechanical loading (TCML) in the chewing simulator were applied simulating 15 years of clinical service. Wear traces were analyzed (frequency [n], depth [µm]) and evaluated using scanning electron pictures. Wear results were compared by one-way-ANOVA and post-hoc-Bonferroni (α = 0.05).
RESULTS
After TCML, no visible wear traces were found on Zr. Li showed more wear traces (n = 30–31) than the reference (n = 21). Antagonistic teeth #26 showed more wear traces in contact to both ceramics (n = 27–29) than to the reference (n = 21). Strong wear traces (> 350 µm) on antagonists and their adjacent teeth were found only in crowned groups. Abutment type influenced number and depth of wear facets on the antagonistic and adjacent teeth.
Full-contour ceramic crowns made of lithium disilicate or zirconia have become very popular due to good esthetics and biocompatibility, as well as their cost-effective computer-aided design and manufacturing (CAD/CAM). These ceramics have proven their suitability for monolithic single crowns in many previous studies.123 Ongoing modifications in ceramic structure and composition (e.g. leucite, lithium silicate, lithium disilicate or lithium-aluminosilicate) offer a wide range of glass-ceramic materials for individual applications according to the clinical requirements of strength (< 500 MPa) and translucency (> 50%). Currently available 3Y-, 4Y- and 5Y- Y2O3 tetragonal zirconia polycrystals (Y-TZP) differ in strength (500 – 1200 MPa) and translucency (< 50%), but show comparable hardness (HV: 1200 – 1300). Hardness and other material properties vary between zirconia (HV: 1200 – 1300), lithium disilicate (HV: 600), and human tooth enamel (HV: 300 – 400).45
However, hard surfaces might not cause increased wear without considering the surface state. Smooth polished and glazed zirconia surfaces did not show high antagonistic wear rates.678 Despite of identified influencing factors (e.g. surface roughness, hardness, occlusal crown design), wear behavior of ceramic restorations in the complex mastication system has not yet been fully understood. The complex interaction between indirect restorations and natural teeth in this system may influence physiological chewing or even provoke craniomandibular dysfunctions. Many previous in vitro studies focused on wear without including the complex influences of the jaw situation.910 Some studies at least evaluated human enamel as antagonistic material.811121314151617 The type of abutment (resilient tooth support or rigid implant situation) may have an impact on wear performance of the crown itself but also on the wear of antagonistic and adjacent teeth. It seems advantageous to design an in vitro model that approximates the in vivo situation with human antagonistic and adjacent teeth in a mastication device simulating relevant clinical parameters (chewing cycles, forces, frequency; thermocycles). Such a clinically approximated two-body wear simulation may allow for comparative evaluation of wear facets and maximum wear on ceramic crown materials and adjacent or antagonistic human teeth, although it may not fully reproduce in vivo wear processes. A comparison to clinical data would be wishful, but clinical wear studies are rare118192021 and valid in vivo methods of evaluation are missing.22
The present pilot study aimed to test the suitability of an in vitro jaw model for wear testing of dental crowns. The hypothesis was that crown material (lithium disilicate, zirconia) and type of abutment (rigid implant, resin tooth with artificial periodontium) have an influence on the wear performance (traces, depth, superficial damages) of molar crowns and their antagonistic and adjacent teeth.
200 extracted human teeth (stored in 0.5% chloramine solution for no longer than four weeks) were selected to mimic 40 models of a clinical posterior situation. For each model, a premolar (mandibular left second premolar: #35) and two molars (mandibular left first molar: #36 and mandibular left second molar: #37) were embedded in resin blocks (Palapress Vario, Kulzer, Hanau, Germany) to simulate a section of the lower jaw. Corresponding human tooth antagonists were selected for the upper jaw (maxillary left second premolar: #25, maxillary left first molar: #26, and maxillary left second molar: #27). The variability of human premolars and molars were respected by preselecting teeth with comparable size and shape and by randomly dividing the teeth to the subgroups. The teeth and their antagonists were positioned in a clinically relevant occlusal contact situation (maximum intercuspidation) using a dental articulator (Artex, Amann-Girrbach, Pforzheim, Germany). Every lower tooth had antagonistic contacts to only one upper tooth. The contact points were adjusted and controlled with articulating paper.
Five groups (see Table 1) with eight models per group were prepared. Two clinically relevant situations (implant- and resin tooth-supported) for restoring tooth the mandibular left first molar (#36) in the lower jaw, as well as two ceramic crown materials (lithium disilicate, zirconia), were investigated. A group with intact human teeth served as reference.
In the two implant (I) groups, the mandibular left first molars (#36) were represented by implant analogues (Straumann, Freiburg, Germany; titanium grade IV, implant diameter 4.1 mm, implant length 12 mm, abutment length 4 mm, 8°), which were rigidly positioned in resin blocks (Palapress Vario) in order to simulate a posterior implant situation.
In the two tooth (T) groups, teeth #36 were represented by identical resin tooth replicas (Palapress Vario), which were flexibly positioned in resin blocks (Palapress Vario) in order to simulate an artificial periodontium. The preparation design was based on ceramic guidelines with a circular reduction of 1 mm and occlusal anatomical reduction of about 1.5 mm (height ~ 6 mm, angle ~ 6°, rounded edges). The 1 mm circumferential deep shoulder with rounded inner angles was at an isogingival height of the tooth cervix.
In the reference (R) group, eight unprepared extracted human teeth #36 with an artificial periodontium were positioned in resin blocks (Palapress Vario).
The resilience of the tooth periodontium (for all human teeth and replaced resin teeth) was simulated by coating the natural or resin roots of the teeth with a 1 mm polyether layer (Impregum, 3M, Neuss, Germany). For achieving a constant layer, the roots were dipped in a wax bath, which was replaced by polyether in the second fabrication process.2324
Mean tooth mobility was 80 µm in the axial direction, 280 µm in the buccal direction, and 130 µm in the oral direction.
In the implant and tooth groups, the implant analogues or resin tooth replicas were restored with lithium disilicate (Li) or zirconia (Zr) crowns, respectively. Therefore, implants and resin teeth were digitalized (Cerec Omnicam, Dentsply Sirona, Bensheim, Germany) and full-contour molar crowns were milled (Cerec, MC XL, Dentsply Sirona; juvenile, spacer 80 µm, contact thickness 25 µm, edge reinforcement 50 µm). Ceramic materials were 3Y-TZP zirconia (IPS e.max ZirCAD, Ivoclar Vivadent, Schaan, Liechtenstein; Mo 1 C15L, LOT: S20285) and lithium disilicate (IPS e.max CAD, Ivoclar Vivadent; LT A2/C14, LOT: T23748) (n = 8 per material per group, Table 1). Zirconia crowns were sintered (Cercon heat plus, Degudent, Hanau, Germany; 1500℃, 2 hours). Lithium disilicate crowns were crystalized and glazed together (820℃; 10 min). All crowns were polished (silicon polisher, diamond paste, EVE Ernst Vetter, Keltern, Germany) before applying a glaze layer and firing in the ceramic furnace (Programat EP 5000, IPS e.max Ceram glaze, Ivoclar Vivadent) following the respective firing protocol provided by the manufacturer. Following this procedure, a consistently smooth and comparably thick glaze layer was achieved. In pretests, the thickness of the glaze layer was determined to range between 100 µm and 120 µm.
The inner surfaces of the zirconia crowns were sandblasted (aluminium oxide, 100 µm, 1.5 bar) and the lithium disilicate crowns were etched for 20 seconds (IPS Ceramic Etching Gel HF, Ivoclar Vivadent). Primer (Monobond-Plus, Ivoclar Vivadent, 60 s) was applied. All crowns on resin teeth were adhesively bonded (Heliobond, Syntac Adhesive, Variolink 2 Catalyst, Variolink Base, Ivoclar Vivadent; Elipar Trilight, 3M). Implants were sandblasted (aluminium oxide, 100 µm, 1.5bar) and primed (Monobond-Plus, 60 s) before bonding the crowns (Multilink Hybrid Abutment, Ivoclar Vivadent; Elipar Trilight, 3M).
Upper and lower restoration models were stored in distilled water until simulation. Thermal cycling and mechanical loading (TC: 6 × 3.000 cycles with changing temperatures between 5℃/55℃, distilled water; ML: 100 N for 3.6 × 106 cycles; f = 1.6 Hz; mouth opening 2 mm; lateral movement 2 mm; chewing simulator EGO, Regensburg, Germany) with online failure-control were performed to simulate and control fatigue failures. Chewing simulation parameters were based on literature data regarding zirconia and ceramic restorations, simulating ten to fifteen years of oral service.2526
After simulation, all teeth and crowns were optically examined (light microscope, Vision Engineering, Woking, UK, 4× magnification) documenting the frequency and location of all wear traces in the area of the contact points. A 3D color laser scanning microscope (VK-X100 Series, Keyence Corporation, Osaka, Japan) was used for quantitative evaluation of the occlusal surfaces. The depth of the respective wear facet was measured, whereby both the deepest point and the average depth were determined. Assuming annual wear rates of about 30 – 35 µm,27 wear depths below 350 µm (after 10 – 15 years of simulation) were graded “normal”. Wear facets deeper than 350 µm were graded “strong”. Exemplary scanning electron micrographs (Quanta FEG 400, FEI Company, Hillsboro, OR, USA; 10 kV, low vacuum, working distance: 30 mm) of tooth #36 and antagonistic tooth #26 in every group were used to investigate crowns and antagonistic surfaces for wear phenomena, defects, or cracks.
Calculations and statistical analyses were performed using SPSS 23.0 for Windows (SPSS Inc., Chicago, IL, USA). Means and standard deviations were calculated and analyzed using one-way analysis of variance (ANOVA) guided by Bonferroni-test for post-hoc analyses where appropriate. The level of significance (α) was set to 0.05.
The number of wear traces was analyzed for all teeth of the upper and lower jaw. Table 2 gives an overview of the total number of wear traces in the different groups (sum of all wear facets of the eight tooth representatives in every model). The number of strong wear facets (> 350 µm) is separately presented.
For the maxillary left second premolar (#25), in total, around twice the number of wear traces (n = 18 – 19) were found on the occlusal surfaces in the crown groups in comparison to the reference tooth group (n = 10). No differences were found between the different types of ceramic crowns (Li/Zr) or the types of abutment (I/T).
For the maxillary left first molar (#26), the total number of wear traces in the crown groups was higher (n = 27 – 29) in comparison to the tooth reference (n = 21). Only crown groups provided strong traces (n = 4 – 12), where Zr-crown groups showed 2 – 3 times higher number of traces (n = 11 – 12) than Li-crown groups (n = 4 – 6) in both comparable implant and resin tooth situations.
For the maxillary left second molar (#27), crown groups and tooth reference showed comparable number of wear traces (n = 20, only I-Li was higher with n = 25). Solely for the crown groups, strong traces (n = 2 – 5) were found. No strong differences were seen between the different types of abutment (I/T).
In summary, for the upper jaw, strongest wear differences between crown systems and the tooth reference were found for the antagonistic maxillary left first molar (#26) followed by minor differences for the maxillary left second premolar (#25).
For the mandibular left second premolar (#35), only group T-Li (n = 11) showed a similar number of wear traces in comparison to the tooth reference situation (n = 13). The other systems provided a higher number of wear traces (n = 19 – 22). Solely for group T-Zr, one strong wear trace was found. An influence of the abutment was found only for Li-crown groups, showing twice the number of wear traces for the implant situation (n = 22).
For the mandibular left first molar (#36), Li crowns in both implant and resin tooth groups showed higher number of wear traces (n = 30 – 31) than the tooth reference (n = 21). All Zr-crowns provided no visible wear traces on the zirconia surface. Strong wear traces were not found in any group. No differences between implant- and resin tooth-supported crowns were seen.
For the mandibular left second molar (#37), all crown groups provided a higher number of wear traces (n = 20 – 32) in comparison to the tooth reference group (n = 15). Distinctly higher results were found for group T-Zr (n = 32). A low number of strong wear traces were determined for all crown groups (n = 1 – 3). Differences between implant and resin tooth groups were found only for Zr.
In conclusion, for the lower jaw, the strongest wear differences between crown groups and the tooth reference group were found for tooth #36, followed by differences for tooth #37.
In general, comparing ceramic crowns and human teeth, ceramic crowns showed different number of wear traces than human teeth. The highest differences were found for crown and antagonistic situations. Only small differences between Li and Zr groups were found. The influence of the type of abutment seems minimal. Figure 1 gives a comparative overview of the total number of wear traces.
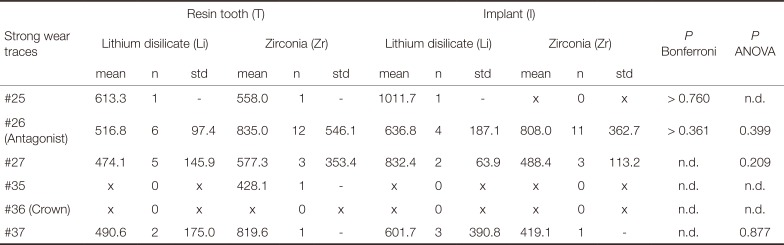
Strong wear traces were quantitatively evaluated. The highest number of strong wear traces was found for the antagonists in the crown groups. Mean depth of strong wear traces of the antagonists (tooth #26) varied between 516.8+/−97.4 µm (T-Li, n = 6), 835.0+/−546.1 µm (T-Zr, n = 12), 636.8+/−187.1 µm (I-Li, n = 4), and 808.0+/−362.7 µm (I-Zr, n = 11). No statistical differences were found between the individual systems (ANOVA: P = .399, Bonferrroni: P > .361). Antagonists against resin tooth-supported Li crowns showed a tendency for lower values in comparison to implant-supported Li crowns.
Due to low numbers of strong wear traces for teeth #25, #27, #35, and #37 statistical differences were not determined. Neither Li nor Zr crowns showed strong wear traces(see Table 2 and Table 3).
Exemplary SEM pictures of worn crown and antagonist surfaces are given in Fig. 2. Reference tooth #36 and its tooth antagonist #26 showed typical wear traces without any cracks or other damages. T-Li and I-Li groups displayed wear traces on both tooth and crown, but no noticeable cracks or damages. For the T-Zr and I-Zr groups, wear traces on the antagonist were found. The zirconia crowns showed slightly worn areas of the glaze layer but no further damage or crack. On the antagonistic surface of T-Zr, a small enamel chipping was found.
The hypothesis that crown material (Li or Zr) and type of abutment (rigid implant, resin tooth with artificial periodontium) have an influence on the wear performance (traces, depth, and superficial damages) of crowns, antagonistic and adjacent teeth was accepted. The amount of wear differed between the Zr and Li crowns and partly between rigid implant or resilient resin tooth, and was influenced by the size of the root surfaces of the adjacent teeth.
While Zr crowns showed wear only in the glaze layer, resulting in the exposure of the underlying zirconia, Li crowns both for the resin tooth and implant situation provided a high number (n = 30 – 31) of wear traces. The number of wear traces on the antagonistic tooth 26 were comparably high (n = 27 – 29) for both ceramic materials and abutment situations, but Zr crown groups showed a 2 – 3 -times higher number of strong traces than Li crown groups. Furthermore, antagonists against resin tooth-supported Li crowns showed lower wear depth values in comparison to implant-supported Li crowns. Generally, adjacent tooth #37 and its antagonistic tooth #27 showed a higher number of strong wear traces than teeth #35 and #25. A tendency to a higher total number of wear traces was found for teeth #35 and #27 of group I-Li, and for tooth #37 of group T-Zr.
Differences between Zr, Li, and the human tooth reference may be attributed to different material properties such as hardness (Li: HV 600, Zr: HV 1200 – 1300, enamel: HV 300 – 400) and flexural strength (Li: < 500 MPa, Zr: 1200 MPa, enamel 300 – 450 MPa). Thus, in contrast to Zr, Li showed wear, as it was reported in many previous studies.72829 Li crowns with wear may be flattened and compensate for chewing forces, resulting in lower loads on adjacent teeth. This effect is enhanced by the intrusion and the lateral movement of teeth with artificial periodontium. In contrast, implant-supported Li crowns have no resilience, resulting in lower force absorbing capacity. As a consequence, adjacent teeth have to bear these additional chewing forces, resulting in increased wear. The larger the root surface, the smaller the expected intrusion of the adjacent teeth. Therefore, tooth #37 is supposed to show less resilience than tooth #35, and consequently the higher number of strong wear traces was found.
Damping effects of implant-supported crowns differ between materials,30 showing the lowest shock absorbing capacity and the highest load transfer for high-strength ceramics like zirconia. As Zr does not abrade itself, a higher force impact on the antagonist may result in increased wear, as it is reflected in a high number of strong antagonistic wear traces, both for resin tooth and implant groups. In the resin tooth group, intrusion of the crowned tooth caused a higher number of wear traces on the adjacent teeth, as the forces were not absorbed by the zirconia crown. In this situation, the size of the root surface may play an important role: the larger the surface, the less the intrusion, resulting in increased wear of adjacent molars compared to premolars. Thus, the significantly higher number of wear traces on tooth #37 in group T-Zr may be explained. In contrast, in group I-Zr, mainly the antagonistic tooth is worn, as the highly wear-resistant Zr in combination with the rigid implant support takes the load off the adjacent teeth.
The results of all groups suggest that the size of the root surface of the adjacent teeth influences their wear behavior. It is assumed that molar teeth with larger root surfaces show less resilience upon chewing forces than premolars, resulting in different absorption processes; molars are able to absorb forces via resilience only to a limited extent, and therefore the enamel layer in the area around the contact points absorbs comparably more chewing forces. This leads to a higher number of strong wear traces. In this context, it has to be considered that a comparison of the total number of wear traces between the different types of teeth is not significant because molars have a larger occlusal surface and consequently a higher number of contact points than premolars.
As the experimental set up of this pilot study did not allow for quantification of tooth intrusion, further studies that investigate the amount and effects of intrusion and consequent load distribution in a complex jaw model are necessary. Furthermore, it cannot be predicted if these observations correspond to the in vivo situation without restrictions since the in vitro simulation in the chewing simulator did not reproduce the three-dimensional structures of the temporomandibular joint. Any possibly occurring compressions of the temporomandibular joint may influence the loading situation of adjacent teeth. Furthermore, different wear behavior of ceramic crown materials compared to the natural teeth might have an influence on occlusion and jaw position or even result in craniomandibular dysfunctions.
Stronger wear traces for antagonists of ceramic crowns than for the reference tooth group were found. Antagonistic wear of Zr was generally higher than that of Li. These results highlight the necessity of a correct occlusal setup and regular recall sessions to control wear behavior and occlusal relation, or make adjustments if required. Long-time clinical studies are needed for a more significant prediction of the wear behavior of ceramic crowns, especially zirconia, with natural antagonistic and adjacent teeth. Currently, there is only limited clinical evidence of enamel wear against zirconia, and adjacent teeth are usually not considered. First in vivo results indicated similar or more antagonistic enamel wear of Zr,11118192021 which corresponded to previous in vitro results.461516 The results indicated the necessity of considering the occlusal setup as well as the antagonistic and adjacent situation in clinical studies.
When comparing implant and resin tooth groups, the rigid positioning of the implants in comparison to the mobile positioning of the resin teeth, associated with the different properties of the abutment materials (modulus of elasticity: resin 2–3 GPa, titanium 110 GPa) or the different preparation geometries, had only limited influence on the wear performance. However, it can be assumed that the geometry and the abutment material can influence the force application and transmission and thus the abrasion behavior. Effects on the adjacent teeth and their antagonists may not be excluded. A limiting factor of the study design is that the artificial periodontal mobility simulates the clinical situation only in rudiments and lacks tactile sensitivity and no proprioceptive motion feedback due to missing periodontal mechanoreceptors. Similarly, resin may not replace the complex bone structure of cortical and cancellous bone, resulting in different damping effects.
Except for small chipping on the antagonistic surface of group T-Zr, no failures or damages were observed by SEM analysis. Chipping as major reason for clinical failure of veneered restorations 313233 was not found in the present study as only monolithic crowns were applied. Based on identical initial surface states (polishing, glazing) of both materials, in the course of the wear simulation, Zr crowns only showed slight worn areas of the glaze layer with exposure of the underlying smooth surface, while Li revealed typical rough wear facets. Wear of glaze and exposure of the underlying ceramic was also shown in previous studies on wear of different ceramics.83435 For Li, removal of the glaze layer or glassy matrix and exposure of crystalline phases has superposed the original surface state. Because of its microstructure and inferior mechanical properties, lithium disilicate is more prone to microploughing, microcracking and microcutting. The roughened surface also provoked wear on the antagonistic teeth, which is reflected in a flattening and a higher number of strong wear traces compared to the tooth reference.
As glaze layers are usually worn within the first months or years of clinical service, the surface treatment of the exposed ceramic may be of high importance. Smooth surfaces are considered to be important for the long-term success of the restoration and reduced wear of the antagonist. However, the hardness of zirconia might be a challenge for polishing. Several studies have dealt with the effect of polishing procedures on surface properties of zirconia.3637383940 Most studies agree that appropriate polishing instruments and procedures are effective in reducing surface roughness.363740 However, severely ground surfaces may not be fully restored by polishing.39 Partly, surface phase changes by polishing were reported.3738 Therefore, careful polishing is recommended to achieve smooth surfaces and to keep phase changes low. Even if glaze layers are gradually worn in contact areas, they are clinically important for aesthetic characterization of crowns and may seal superficial pores and cracks in the ceramic surface. The glaze may also protect the zirconia surface from aging effects and low temperature degradation in the moist oral cavity. Furthermore, the glaze layer may be necessary for some favourable “fitting wear”,8 as it has to be considered that human teeth are also exposed to continuous height changes by wear under clinical conditions, which were reported to range between 30 and 40 µm per year.27
Potential effects of the humid oral environment on ceramic wear and aging were considered by thermocycling with water. Cyclic loading in water combined with temperature changes (5℃/55℃) might induce subcritical crack propagation and low temperature degradation (LTD) of zirconia, which may influence mechanical properties and wear.414243
For a clinically relevant wear simulation and evaluation, human antagonistic and adjacent teeth were used. Although teeth with comparable size and shape were selected, individual differences in morphology, differences in surface state and roughness, thickness and hardness of the enamel layer among other mechanical factors might account for a broader distribution of results. Optimal contact situations were achieved by a tooth-to-tooth situation with only one antagonist per tooth. Nevertheless, different number and distribution of contact points due to differences in the tooth morphology might have influenced loading and consequent wear. However, these variations in human teeth reflect the in vivo situation more realistically than artificial teeth with standardized geometry and composition. As the highest variations in the enamel structure as well as predamage are expected primarily in the superficial enamel layer, evaluation of the wear depth allowed for better comparison between the groups than wear volume or area. Due to the lack of standardization in wear evaluation methods, comparison to other studies is difficult and determination of general reference values for (strong) wear is not possible. Therefore, after evaluation of all wear data and comparison to the tooth reference group in this study, we defined a threshold value for wear (350 µm) that allowed a differentiation between the total number of wear facets and strong wear. Assuming annual wear rates of about 30 – 35 µm,27 wear depths below 350 µm (after 10 – 15 years of simulation) were graded “normal”. Strong wear facets (> 350 µm) were further analyzed, and although their number varied among the groups, the values were in a realistic range (antagonists of crowns: about 500 – 800 µm). Especially for groups with a similar total number of wear facets, the number of strong wear facets allowed to clearly identify differences in the severity of wear (e.g. tooth #26: T-Li versus T-Zr, I-Li versus I-Zr). As there were high variations in wear data due to individual tooth differences, this global differentiation of wear under consideration of all tooth representatives of one group is more significant than comparing individual wear values of every single tooth.
Wear performance of monolithic ceramic molar crowns was evaluated in a clinically relevant model with human antagonistic and adjacent teeth. Wear depths and the number of wear traces differed between Zr and Li crowns, showing worn glaze with exposure of the underlying smooth surface for Zr, and deep wear facets for Li.
The crown material in combination with the type of abutment (implant, resin tooth) influenced the number of wear traces and the amount of strong wear (> 350 µm) of antagonistic and adjacent teeth. Antagonists against Zr showed about twice the amount of strong wear traces than Li irrespective of the abutment situation. Adjacent molar teeth of crowns and their antagonists revealed a higher amount of strong wear traces than adjacent premolar teeth due to differences in the size of the root surfaces that influence their ability of intrusion. The results highlight the necessity of a correct occlusal setup and regular recall sessions to control wear behavior and occlusal relation.
Based on the results of this pilot study, the principal suitability of the applied in vitro jaw model for wear testing was shown. A limited comparative evaluation of the wear situation was enabled. Further development of the experimental set-up (e.g. measurement of tooth intrusion and load distribution) is recommended.
References
1. Pathan MS, Kheur MG, Patankar AH, Kheur SM. Assessment of antagonist enamel wear and clinical performance of full-contour monolithic zirconia crowns: One-year results of a prospective study. J Prosthodont. 2019; 28:e411–e416. PMID: 30256495.

2. Rabel K, Spies BC, Pieralli S, Vach K, Kohal RJ. The clinical performance of all-ceramic implant-supported single crowns: A systematic review and meta-analysis. Clin Oral Implants Res. 2018; 29:196–223. PMID: 30306684.

3. Rauch A, Reich S, Dalchau L, Schierz O. Clinical survival of chair-side generated monolithic lithium disilicate crowns:10-year results. Clin Oral Investig. 2018; 22:1763–1769.

4. Ludovichetti FS, Trindade FZ, Werner A, Kleverlaan CJ, Fonseca RG. Wear resistance and abrasiveness of CAD-CAM monolithic materials. J Prosthet Dent. 2018; 120:318.e1–318.e8. PMID: 30097264.

5. Hayashi S, Homma S, Takanashi T, Hirano T, Yoshinari M, Yajima Y. Wear properties of esthetic dental materials against translucent zirconia. Dent Mater J. 2019; 38:250–256. PMID: 30541995.

6. Chong BJ, Thangavel AK, Rolton SB, Guazzato M, Klineberg IJ. Clinical and laboratory surface finishing procedures for zirconia on opposing human enamel wear: A laboratory study. J Mech Behav Biomed Mater. 2015; 50:93–103. PMID: 26116957.

7. Preis V, Grumser K, Schneider-Feyrer S, Behr M, Rosentritt M. Cycle-dependent in vitro wear performance of dental ceramics after clinical surface treatments. J Mech Behav Biomed Mater. 2016; 53:49–58. PMID: 26313248.

8. Preis V, Behr M, Handel G, Schneider-Feyrer S, Hahnel S, Rosentritt M. Wear performance of dental ceramics after grinding and polishing treatments. J Mech Behav Biomed Mater. 2012; 10:13–22. PMID: 22520415.

9. D'Arcangelo C, Vanini L, Rondoni GD, Vadini M, De Angelis F. Wear evaluation of prosthetic materials opposing themselves. Oper Dent. 2018; 43:38–50. PMID: 28857711.
10. D'Arcangelo C, Vanini L, Rondoni GD, De Angelis F. Wear properties of dental ceramics and porcelains compared with human enamel. J Prosthet Dent. 2016; 115:350–355. PMID: 26553254.
11. Gou M, Chen H, Kang J, Wang H. Antagonist enamel wear of tooth-supported monolithic zirconia posterior crowns in vivo: A systematic review. J Prosthet Dent. 2019; 121:598–603. PMID: 30509545.
12. Wiegand A, Credé A, Tschammler C, Attin T, Tauböck TT. Enamel wear by antagonistic restorative materials under erosive conditions. Clin Oral Investig. 2017; 21:2689–2693.

13. Fathy SM, Swain MV. In-vitro wear of natural tooth surface opposed with zirconia reinforced lithium silicate glass ceramic after accelerated ageing. Dent Mater. 2018; 34:551–559. PMID: 29361309.

14. Zheng J, Zeng Y, Wen J, Zheng L, Zhou Z. Impact wear behavior of human tooth enamel under simulated chewing conditions. J Mech Behav Biomed Mater. 2016; 62:119–127. PMID: 27183431.

15. Nakashima J, Taira Y, Sawase T. In vitro wear of four ceramic materials and human enamel on enamel antagonist. Eur J Oral Sci. 2016; 124:295–300. PMID: 27059093.

16. Zandparsa R, El Huni RM, Hirayama H, Johnson MI. Effect of different dental ceramic systems on the wear of human enamel: An in vitro study. J Prosthet Dent. 2016; 115:230–237. PMID: 26548885.
17. Lee A, Swain M, He L, Lyons K. Wear behavior of human enamel against lithium disilicate glass ceramic and type III gold. J Prosthet Dent. 2014; 112:1399–1405. PMID: 25311791.

18. Hartkamp O, Lohbauer U, Reich S. Antagonist wear by polished zirconia crowns. Int J Comput Dent. 2017; 20:263–274. PMID: 28852744.
19. Esquivel-Upshaw JF, Kim MJ, Hsu SM, Abdulhameed N, Jenkins R, Neal D, Ren F, Clark AE. Randomized clinical study of wear of enamel antagonists against polished monolithic zirconia crowns. J Dent. 2018; 68:19–27. PMID: 29042241.

20. Yang SW, Kim JE, Shin Y, Shim JS, Kim JH. Enamel wear and aging of translucent zirconias: In vitro and clinical studies. J Prosthet Dent. 2019; 121:417–425. PMID: 30391060.
21. Lohbauer U, Reich S. Antagonist wear of monolithic zirconia crowns after 2 years. Clin Oral Investig. 2017; 21:1165–1172.
22. Rashid H, Sheikh Z, Misbahuddin S, Kazmi MR, Qureshi S, Uddin MZ. Advancements in all-ceramics for dental restorations and their effect on the wear of opposing dentition. Eur J Dent. 2016; 10:583–588. PMID: 28042280.

23. Rosentritt M, Behr M, Scharnagl P, Handel G, Kolbeck C. Influence of resilient support of abutment teeth on fracture resistance of all-ceramic fixed partial dentures: an in vitro study. Int J Prosthodont. 2011; 24:465–468. PMID: 21909489.
24. Scharnagl P, Behr M, Rosentritt M, Leibrock A, Handel G. Simulation of physiological tooth mobility in in-vitro stress examination of dental restorations in the masticator. J Dent Res. 1998; 77:1260.
25. Rosentritt M, Behr M, van der Zel JM, Feilzer AJ. Approach for valuating the influence of laboratory simulation. Dent Mater. 2009; 25:348–352. PMID: 18829097.

26. Rosentritt M, Siavikis G, Behr M, Kolbeck C, Handel G. Approach for valuating the significance of laboratory simulation. J Dent. 2008; 36:1048–1053. PMID: 18848380.

27. Lambrechts P, Braem M, Vuylsteke-Wauters M, Vanherle G. Quantitative in vivo wear of human enamel. J Dent Res. 1989; 68:1752–1754. PMID: 2600255.

28. Preis V, Weiser F, Handel G, Rosentritt M. Wear performance of monolithic dental ceramics with different surface treatments. Quintessence Int. 2013; 44:393–405.
29. Lawson NC, Janyavula S, Syklawer S, McLaren EA, Burgess JO. Wear of enamel opposing zirconia and lithium disilicate after adjustment, polishing and glazing. J Dent. 2014; 42:1586–1591. PMID: 25257823.

30. Rosentritt M, Schneider-Feyrer S, Behr M, Preis V. In vitro shock absorption tests on implant-supported crowns: Influence of crown materials and luting agents. Int J Oral Maxillofac Implants. 2018; 33:116–122. PMID: 28518187.

31. Tsumita M, Kokubo Y, Ohkubo C, Sakurai S, Fukushima S. Clinical evaluation of posterior all-ceramic FPDs (Cercon): a prospective clinical pilot study. J Prosthodont Res. 2010; 54:102–105. PMID: 20117970.

32. Teichmann M, Wienert AL, Rückbeil M, Weber V, Wolfart S, Edelhoff D. Ten-year survival and chipping rates and clinical quality grading of zirconia-based fixed dental prostheses. Clin Oral Investig. 2018; 22:2905–2915.

33. Schmitter M, Mueller D, Rues S. Chipping behaviour of all-ceramic crowns with zirconia framework and CAD/CAM manufactured veneer. J Dent. 2012; 40:154–162. PMID: 22197634.

34. Rosentritt M, Preis V, Behr M, Hahnel S, Handel G, Kolbeck C. Two-body wear of dental porcelain and substructure oxide ceramics. Clin Oral Investig. 2012; 16:935–943.

35. Preis V, Behr M, Kolbeck C, Hahnel S, Handel G, Rosentritt M. Wear performance of substructure ceramics and veneering porcelains. Dent Mater. 2011; 27:796–804. PMID: 21524788.

36. Preis V, Grumser K, Schneider-Feyrer S, Behr M, Rosentritt M. The effectiveness of polishing kits: influence on surface roughness of zirconia. Int J Prosthodont. 2015; 28:149–151. PMID: 25822299.

37. Preis V, Schmalzbauer M, Bougeard D, Schneider-Feyrer S, Rosentritt M. Surface properties of monolithic zirconia after dental adjustment treatments and in vitro wear simulation. J Dent. 2015; 43:133–139. PMID: 25174949.

38. Bartolo D, Cassar G, Al-Haj Husain N, Özcan M, Camilleri J. Effect of polishing procedures and hydrothermal aging on wear characteristics and phase transformation of zirconium dioxide. J Prosthet Dent. 2017; 117:545–551. PMID: 27881307.

39. Al-Haj Husain N, Camilleri J, Özcan M. Effect of polishing instruments and polishing regimens on surface topography and phase transformation of monolithic zirconia: An evaluation with XPS and XRD analysis. J Mech Behav Biomed Mater. 2016; 64:104–112. PMID: 27497266.

40. Al-Haj Husain N, Özcan M. A study on topographical properties and surface wettability of monolithic zirconia after use of diverse polishing instruments with different surface coatings. J Prosthodont. 2018; 27:429–442. PMID: 27469615.

41. Alghazzawi TF, Lemons J, Liu PR, Essig ME, Bartolucci AA, Janowski GM. Influence of low-temperature environmental exposure on the mechanical properties and structural stability of dental zirconia. J Prosthodont. 2012; 21:363–369. PMID: 22372432.

42. Salazar Marocho SM, Studart AR, Bottino MA, Bona AD. Mechanical strength and subcritical crack growth under wet cyclic loading of glass-infiltrated dental ceramics. Dent Mater. 2010; 26:483–490. PMID: 20303160.

43. Zhang Y, Song JK, Lawn BR. Deep-penetrating conical cracks in brittle layers from hydraulic cyclic contact. J Biomed Mater Res B Appl Biomater. 2005; 73:186–193. PMID: 15672403.

Fig. 1
Overview of total number of wear traces on crown 36, antagonist 26, and adjacent teeth (25, 27, 35, 37) in the different groups.
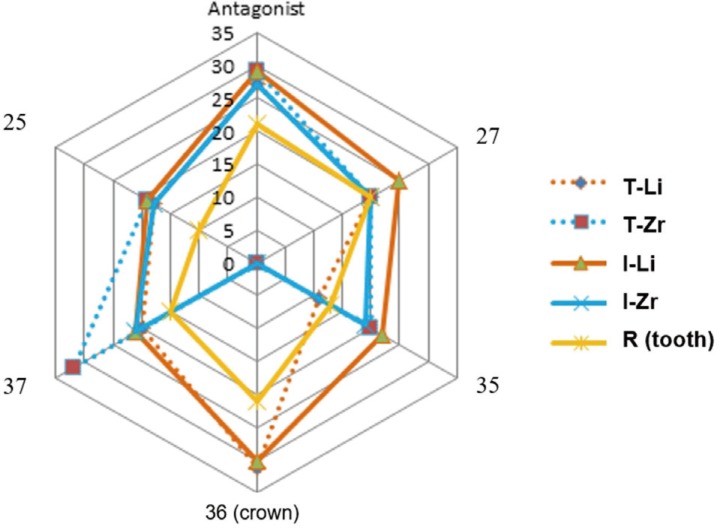
Fig. 2
Exemplary SEM pictures of worn crown surfaces and corresponding antagonistic enamel wear facets: (A) Occlusal surface of Li crown (30× magnification), (B) Wear facet of tooth antagonist of Li crown (100× magnification), (C) Occlusal surface of Zr crown (30× magnification), (D) Wear facet of tooth antagonist of Zr crown (100× magnification).
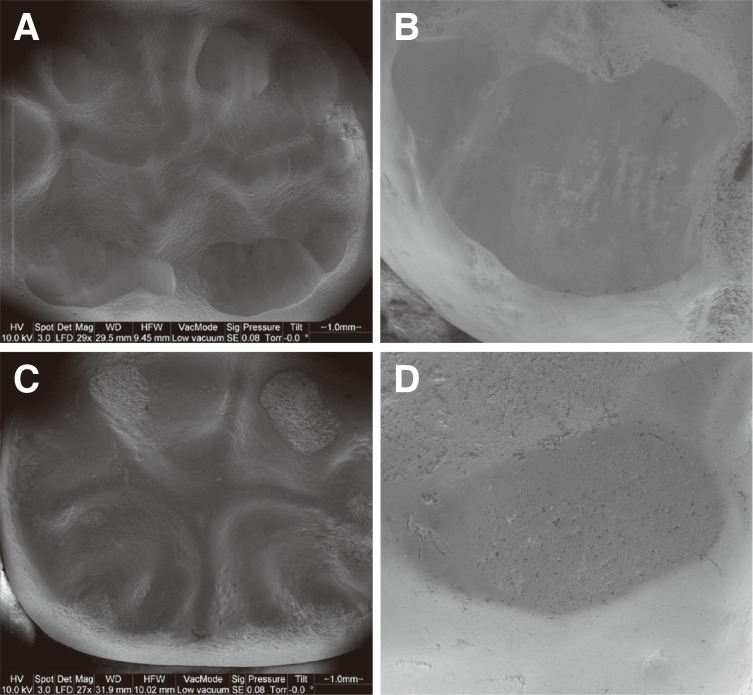
Table 1
Study overview (5 groups)
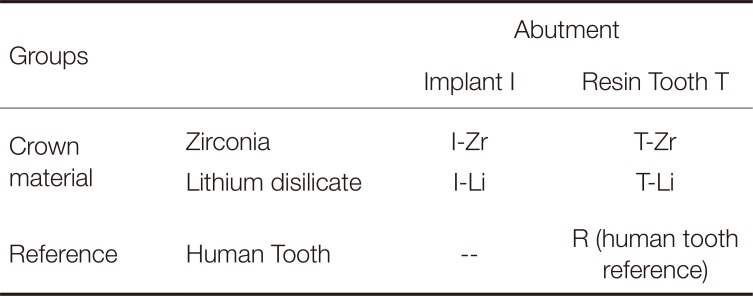
| Groups | Abutment | ||
|---|---|---|---|
| Implant I | Resin Tooth T | ||
| Crown material | Zirconia | I-Zr | T-Zr |
| Lithium disilicate | I-Li | T-Li | |
| Reference | Human Tooth | -- | R (human tooth reference) |
Table 2
Number of wear traces (sum total, strong traces; *: human tooth) of groups differing in crown material (lithium disilicate, zirconia) and abutment situation (resin tooth, implant)
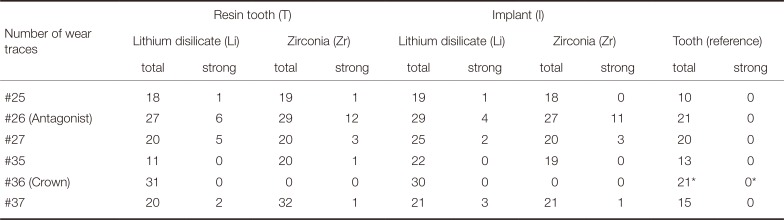
Table 3
Strong wear traces




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download