INTRODUCTION
Breast adenomyoepitheliomas are biphasic tumors composed of epithelial and myoepithelial cells forming tubules, lobules, or solid structures. Adenomyoepitheliomas typically present as a solitary palpable nodule ranging from 1 to 7 cm in size [
12]. They are circumscribed but may have lobulated, slightly irregular, pushing borders. Although these heterogeneous tumors are considered to be benign or have low malignant potential, transformation of one or both cellular components may occur with the development of metastatic disease [
12]. According to Lubin et al. [
3], malignant tumors exhibit severe cytologic atypia, an infiltrative growth pattern, and an increased amount of mitosis (> 3/10 high-power field) or necrosis. The molecular events associated with malignant adenomyoepitheliomas are poorly understood due to their rarity and the limited data about them [
234].
Malignant adenomyoepitheliomas may exhibit similar morphological and immunophenotypic features like those of basal-like breast cancers, metaplastic breast carcinomas, and epithelial-myoepithelial carcinomas (EMC) of the salivary gland [
1345]. Interestingly, few molecular alterations have been associated with progression from the
in situ to the invasive stage of breast carcinoma. For example, gene amplification of
MYC on chromosome 8q24 is detected in the invasive component of
MYC-amplified breast cancers, but is absent in the intraductal component [
6]. Therefore, deregulation of
MYC appears to be essential in the acquisition of a malignant phenotype in breast cancers [
7]. Here, we report for the first time, a malignant adenomyoepithelioma overexpressing c-MYC due to
MYC amplification, an observation that was absent in the benign neoplastic elements. Gene amplification events could be crucial in facilitating cancer progression in adenomyoepitheliomas.
CASE REPORT
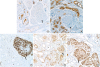
An otherwise healthy, 47-year-old woman visited for surgical consultation after a 2.8 cm solid mass was found during a routine screening mammogram. The mass was located in the left, lower, inner quadrant and was radiologically categorized as BI-RADS 4 (suspicious for malignancy). The biopsy diagnosis was consistent with an adenomyoepithelioma. One month later, a partial mastectomy was performed. Pathological examination revealed a neoplasm growing with pushing borders, composed of benign and malignant components (
Figure 1). The benign component consisted of tubular structures lined by epithelial cells with scant eosinophilic cytoplasm, small hyperchromatic nuclei, and surrounding myoepithelial cells with clear cytoplasm (
Figure 2A-E). The malignant component consisted of solid nests and islands of carcinoma cells with enlarged nuclei, multiple small nucleoli, a high nuclear-cytoplasmic ratio, and numerous mitotic figures, which were also seen invading non-neoplastic breast tissue (
Figure 2F-J).
Figure 1
Macroscopic and microscopic pathology of the breast adenomyoepithelioma. (A) Gross (scale bar = 1 cm) and microscopic pathology of a breast adenomyoepithelioma with (B) a benign component (asterisk) showing predominant tubular architecture growing with pushing borders (H&E staining, × 25), and (C) a carcinomatous component (arrow) growing in nests, islands, and a few cribriform structures invading breast tissue on the right (H&E staining, × 25).

Figure 2
Staining of the adenomyoepithelioma for markers of epithelial and myoepithelial differentiation. Epithelial and myoepithelial differentiation in the benign (A-E) and malignant (F-J) components of the adenomyoepithelioma. Epithelial elements were positive for CAM5.2 (B, G) and AR (C, H). Myoepithelial cells were positive for p63 (D, I) and SMMHC (E, J), but negative for CAM5.2 and AR. All microphotographs are shown at high magnification (× 400).
AR = androgen receptor.

These observations are consistent with a carcinoma arising from an adenomyoepithelioma. The biphasic nature of the adenomyoepithelioma, demonstrated morphologically by epithelial and myoepithelial cells arranged in tubules, was lost in the malignant component. Although the malignant cellular proliferation presented as a conglomeration of highly atypical cells with no morphological clues of differentiation, the carcinoma still exhibited epithelial and myoepithelial differentiation by immunohistochemistry (
Figure 2F-J). The epithelial cells were strongly positive for CAM5.2 cytokeratin (Dickinson, Becton Lakes, USA) and androgen receptor (AR; Cell Marque, Rocklin, USA), but negative for myoepithelial markers including smooth muscle myosin heavy chain (SMMHC; Ventana, Tucson, USA), α-smooth muscle actin (sm-actin-1A4; Ventana), p63 (Cell Marque), and CK5/6 (Cell Marque). In contrast, the myoepithelial cells were strongly positive for SMMHC, α-smooth muscle actin, high molecular weight cytokeratin including CK5/6 and CK34βE12 (Dickinson), SOX-10 (Biocare, Pacheco, USA), S100 (Cell Marque), and p63, but very weak for CAM5.2 and negative for AR.
Notably, the benign epithelial cells showed strong immunoreactivity for carcinoembryonic antigen (Dickinson) and epithelial membrane antigen (Dickinson) in a membranous apical pattern, both of which were lost in the carcinoma cells (
Figure 3A and B). GATA-3 (Ventana) was observed to be strongly positive in foci within the benign tubules but weak within the carcinoma. The ki-67 proliferation index was high in the malignant component but low in the benign component (
Figure 3C). Estrogen and progesterone receptors (ER, PR) and HER-2/neu were negative, and cyclin-D1 (
Figure 3D) was positive in both the benign and malignant components. The tumor was also screened for p16
INK4a, p53, and c-MYC abnormalities. p53 showed immunoreactivity to wild type levels in both components, ruling out mutations in
TP53. We observed variable expression of p16
INK4a in both components, suggestive of cellular senescence and therefore ruling out homozygous deletion of
CDKN2A. Overexpression of c-MYC was detected only in the carcinoma cells, with immunoreactivity covering both areas showing epithelial and myoepithelial differentiation (
Figure 3E).
Figure 3
Immunohistochemical changes associated with malignant transformation in the adenomyoepithelioma. (A, B) Loss of expression of (A) epithelial membrane antigen and (B) carcinoembryonic antigen. (C) Ki-67 labeling showing a high proliferation index in the carcinoma (80%) as compared with benign tubules (< 1%). (D) Diffuse nuclear expression of cyclin D1 in both components, suggestive of early oncogenic alterations in the mitogen-activated protein kinase pathway. (E) Diffuse and strong expression of c-MYC in the carcinoma, including the epithelial and myoepithelial malignant cells. No expression was observed in the benign tubules. Magnifications used: × 400 for (A), × 200 for (B-E). For all panels: benign components are marked with an asterisk, and malignant components are marked with an arrow.
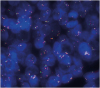
Break-apart fluorescence
in situ hybridization was performed on formalin-fixed paraffin-embedded sections of the tumor with a mixture of
MYC spectrum orange and green probes (Abbott Laboratories, Des Plaines, USA). The normal pattern of observations is 2 fusion events per cell (2 F/cell). However, in the tumor, we observed abnormal signal patterns (3–4 F/cell in 37%, > 4 F/cell in 40%, and 0 F/cell in 7%) indicating amplification of the
MYC gene region on chromosome 8q24 and no evidence of rearrangement (
Figure 4). After discussion with our Multidisciplinary Breast Tumor Board, the patient underwent re-excision of the surgical site due to the presence of positive margins. Subsequently, the surgical margins were cleared of tumor and no chemoradiotherapy was administered. After one year of follow up, the patient has remained free of disease with no evidence of local or distant recurrence.
Figure 4
Break-apart fluorescence in situ hybridization analysis of carcinoma cells showing abnormal signal patterns. The number of fusions per cell was measured. Normal pattern is 2 F/cell, but carcinoma cells presented with: 3–4 F/cell in 37% of cells, > 4 F/cell in 40%, and 0 F/cell in 7%. This observation indicates amplification of the MYC gene region on chromosome 8q24 and no evidence of rearrangement.
DISCUSSION
Identification of a malignant component within adenomyoepitheliomas is of utmost clinical relevance because these tumors can relapse and disseminate, leading to a worsened outcome [
1]. There are reports of metaplastic breast carcinoma, EMC, and adenoid cystic carcinoma in association with breast adenomyoepitheliomas [
234]. However, the malignant component of the adenomyoepithelioma may not display distinctive architectural patterns to meet the criteria for a particular subtype of breast cancer. As in the present case, demonstration of myoepithelial and epithelial differentiation by immunohistochemistry can help highlight the biphasic nature of malignant adenomyoepitheliomas and rule out other more common breast carcinomas, especially those with a basal-like immunophenotype [
2]. Adenomyoepitheliomas belong to a broad category of biphasic epithelial-myoepithelial proliferations. Interestingly, adenomyoepitheliomas share the same morphological, immunophenotypic, and molecular features as EMC in the salivary gland [
3]. Both types cover a broad spectrum of completely benign tumors to clinically aggressive carcinomas. Some authors have even suggested that benign adenomyoepitheliomas should be called epithelial-myoepithelial adenoma [
8]. Other authors have used the term “adenomyoepithelioma with EMC transformation” when the malignant component displays biphasic differentiation [
59]; however, this designation is not typically used when the malignant component is monophasic or undifferentiated. According to the 2012 World Health Organization classification of breast tumors, the term “adenomyoepithelioma with carcinoma” is preferred [
10].
Breast adenomyoepitheliomas tend to be classified based on their ER status, but malignancy can be observed in both ER-positive and ER-negative tumors. Next-generation sequencing studies have shown that adenomyoepitheliomas harbor somatic mutations that are oncogenic drivers in members of the phosphoinositide 3-kinase (PI-3K) pathway, such as
PIK3CA,
AKT1, and
PIK3R1, as well as in members of the mitogen-activated protein kinase (MAPK) pathway, including the hotspot
HRAS
Q61R mutation [
34]. Concurrent abnormalities in both pathways are uncommon in the duct cell type of breast cancer, but they have been reported in adenomyoepitheliomas with EMC [
5]. Despite the fact that mutations were not tested for in the current case, the diffuse expression of cyclin D1 (a downstream effector of the MAPK pathway) suggests that the benign and malignant components harbored a genetic alteration leading to hyperactivation of the MAPK pathway.
Hotspot mutations in the PI-3K and MAPK pathways are found in either benign or malignant adenomyoepitheliomas. Therefore, they do not explain why these tumors undergo malignant transformation. Geyer et al. [
4] reported hotspot mutations of the telomerase reverse transcriptase (
TERT) gene and homozygous deletion of
CDKN2A (p16
INK4a) associated with carcinomas arising in adenomyoepitheliomas. In another study, by Lubin et al. [
3], malignant adenomyoepitheliomas did not exhibit any pathognomonic non-synonymous mutations compared to their benign counterparts; however, only mutations in 50 cancer-related genes were interrogated. A broad range of genomic and epigenetic alterations could explain the process of malignant transformation in these biphasic neoplasms.
In this case study, we have identified overexpression and amplification of the
MYC gene involved in the carcinomatous transformation of an otherwise benign adenomyoepithelioma.
MYC is a multifunctional oncogene located on human chromosome 8q24.21, and is involved in the regulation of cellular growth, proliferation, metabolism, differentiation, and apoptosis [
67].
MYC has been reported to be amplified and overexpressed in a variety of cancers, and it appears to be an essential step in the progression to invasion [
6].
MYC amplification has been found in approximately 15% of breast cancers, whereas protein overexpression is seen in up to 40% of breast cancer cases [
7]. As a downstream effector of ER and epidermal growth factor receptor family pathways, overexpression of c-MYC may contribute to resistance to adjuvant therapy.
MYC amplification is particularly enriched in tumors with a basal-like phenotype and accounts for the acquisition of cancer stem cell-like properties and the promotion of epithelial-to-mesenchymal transition, which is associated with poor prognosis, high histopathological grades, and tumor progression [
7]. Of note, BRCA-1-inactivated breast cancers, through either mutation or promoter hypermethylation, are prone to develop
MYC amplification [
11]. Therefore, genomic instability probably precedes amplification of the
MYC gene early in carcinogenesis.
We propose that c-MYC overexpression was one of the mediators that contributed to the transition to a fully invasive phenotype in the adenomyoepithelioma described above. Immunostaining patterns for p16
INK4a and p53 ruled out mutations or homozygous deletions of these tumor suppressor genes. However, co-occurring mutations in
TERT and other oncogenes may still be present, leading to cellular transformation. The pivotal role of c-MYC in the development of breast cancer is linked to its numerous genomic targets. The expression of cyclin D proteins and cyclin-dependent kinases, as well as the repression of p21
CIP1 and E-cadherin, are needed for c-MYC-mediated tumorigenesis, including uncontrolled cellular proliferation [
7]. Cell immortalization is explained by inducing the expression of
TERT. Indeed, mRNA levels of
MYC and
TERT correlate in breast cancers [
12]. These data support the hypothesis of malignant transformation associated with c-MYC overexpression. To the best of our knowledge, this is the first study to report
MYC amplification and overexpression in a breast adenomyoepithelioma or an EMC from any location in the body. Further studies should explore the role of gene amplification events in the pathogenesis of malignant adenomyoepitheliomas.








 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download