This article has been
cited by other articles in ScienceCentral.
Abstract
Minimal invasive surgical technique has been increasingly applied to breast surgery. Since the first robot-assisted nipple-sparing mastectomy was introduced, we have been performing nipple-sparing mastectomy using multi-port robotic surgical system. Last year, the new robotic surgical system with single port was introduced. We report the development of a robotic nipple-sparing mastectomy with immediate reconstruction through a single incision using the updated single-port surgical robot system for a patient with ductal carcinoma in situ (DCIS). Breast reconstruction was performed using implants. Postoperative pathological examination revealed DCIS in both breasts. There were no major immediate complications, except for a minor skin burn on the right breast. Overall, the initial operation using the updated platform was safely performed.
Keywords: Breast neoplasms, Mastectomy, subcutaneous, Robotic surgical procedures, Minimally invasive surgical procedures, Mammaplasty
INTRODUCTION
Robotic mastectomy was first introduced by Toesca et al. [
1] in 2015. Since then, various robotic techniques in breast surgery have been developed. Gas-inflated robotic mastectomy using the multi-port da Vinci Xi
® system (Intuitive Surgical, Sunnyvale, USA) was first performed by Sarfati et al. [
2] in 2015. Gasless robotic mastectomy using the multi-port system was also first attempted by Park et al. [
3] in 2016. These robotic mastectomies using a multi-port system had similar basic surgical principles: a 2.5–6-cm axillary incision, manual sentinel lymph node biopsy via the axillary incision, use of tumescent solution for hydrodissection, use of monopolar scissors or spatula for flap dissection, intraoperative evaluation of the nipple areolar complex in patients with breast cancer, and immediate prosthetic reconstruction including direct-to-implant or tissue expander insertion methods [
3456].
However, there are some technical difficulties in robotic mastectomy using multi-port systems. In particular, collisions between robotic arms, collisions between robotic and patient arms, difficulty in use of the third robotic arm, and existence of blind spots due to the rigid 3-dimensional camera scope are potential challenges observed in the initial experience of robotic mastectomy using multi-port systems. However, robotic mastectomy has been rapidly adopted by several surgeons, even among those with no experience in endoscopic surgery.
To overcome these hurdles, a single-port (SP) surgical robot system was developed in 2018. The updated SP surgical robot system, da Vinci SP® (Intuitive Surgical), provides better experience for surgeons with a flexible 3-dimensional camera and robotic arms with a wider range of motion than previous versions of the multi-port surgical robot systems.
Before the clinical application of the updated robotic surgical system, a pre-clinical cadaveric study was performed before applying the system to patients in Medizin im Grünen, Berlin, Germany in 2018. Two surgeons experienced in robotic mastectomy participated in the cadaveric study. To use the SP system for mastectomy, gel-type SP devices manufactured by a third-party, Alexis® O Wound Protector and GelPoint® (Applied Medical, Rancho Santa Margarita, USA) were inserted into the working space. All procedures were safely performed without any skin injury or other organ damage.
Herein, we report for the first time the development of a robotic nipple-sparing mastectomy (RNSM) with immediate reconstruction using the updated platform.
CASE REPORT
After successful application of the SP system for nipple-sparing mastectomy for 6 breasts of 3 cadavers, the first clinical case of RNSM using the SP system was performed. This case report was approved by the Institutional Review Board at Severance Hospital (4-2019-0449). The written informed consent of the operation was taken from the patient before the surgery. A 37-year-old woman visited Severance Hospital in October 2018. Ductal carcinoma
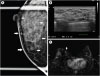
in situ (DCIS) was detected through screening at a local clinic. Preoperative imaging investigations included mammography, breast ultrasound imaging, and breast magnetic resonance imaging (MRI). Mammography revealed that there was a 6.3-cm segmentally distributed suspicious microcalcification at the mediocentral part of the right breast (
Figure 1A). There were non-mass lesions of about 2.2 cm wide with internal calcification extending to the subareolar area at the 2–3 o'clock position of the right breast in the breast ultrasound (
Figure 1B). Additionally, there was a 0.6-cm low suspicious finding at the 10 o'clock position of the right breast in the breast ultrasound examination. Breast MRI revealed a suspicious clumped enhancement in the upper medial to the mediocentral part of the right breast (
Figure 1C). There was no evidence of distant metastasis in whole body bone scan, chest computed tomography, abdomen ultrasound, or evaluation of tumor markers including carcinoembryonic antigen and cancer antigen 15-3.
Figure 1
Images of the patient's preoperative evaluation. (A) Mammography magnification of the right breast showed 6.3-cm segmentally distributed suspicious microcalcification toward the nipple. (B) A 2.2-cm non-mass lesion with internal calcification extended to the subareolar area at the 2–3' o clock position of the right breast in the ultrasound examination. (C) Magnetic resonance imaging showed suspicious clumped enhancement in the upper medial to mediocentral of the right breast.
There was no remarkable past or family history of breast cancer. Because the patient was young, genetic counseling was provided by a physician with certification in genetic counseling. Although a germline BRCA mutation was not detected by the genetic test, the patient wanted to remove her contralateral breast due to severe anxiety. After careful shared discussion about the advantages and disadvantages of contralateral prophylactic mastectomy, she decided to undergo this procedure at the time of definitive surgery for the ipsilateral breast with DCIS.
Bilateral robotic mastectomy using the SP system was performed on November 29, 2018. Before the surgery, a radioisotope for sentinel lymph node biopsy was injected into the periareolar area of the right breast. Left-sided mastectomy was performed first. The patient was placed in the supine position with the arm in the abduction position. A shoulder pad was placed under her back. Before the incision, breast borders were marked using a blue dye (Carmine injection 0.8%
®; Korea United Pharm Inc., Seoul, Korea). The initial incision was located between the anterior and midaxillary line. The vertical incision was parallel to the axillary line and at the level of the nipple. The initial incision size for the left breast was 2.7 cm (
Figure 2A). After the skin incision, the working space was prepared by manual dissection using electrocautery. After the working space was prepared, the tumescent solution was injected into the subcutaneous tissue of the breast. The subcutaneous flap was tunneled using Metzenbaum scissors.
Figure 2
The pictures of processes of robot-assisted nipple-sparing mastectomy using the single-port system. (A) A 2.7-cm single incision was made for the left breast. (B) The patient cart was placed at the opposite side of the operation field.
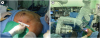
An Alexis
® O wound protector was placed in the working space. A cannula of the robotic system was inserted to the GelPoint
® that was attached to the wound protector. The patient cart was placed at the opposite side of the operation field (
Figure 2B). The docking was performed by the operator. A camera was installed in the camera port. Fenestrated bipolar forceps (FBF), Maryland forceps, and monopolar curved scissors (MCS) were mounted on arms 1, 2, and 3, respectively. The docking time was 5 minutes.
The robotic arms were controlled by the surgeon with the console. Subcutaneous flap dissection to the medial border of the breast was performed using MCS with counter-retracting using FBF. The camera stood in the cobra position for the medial subcutaneous dissection. When the blue dye was observed along the medial border, the dissection was stopped. The upper and lower subcutaneous flap dissections were performed in a similar manner. After the completion of the subcutaneous flap dissections, instrument drives were rotated 180° and the camera drive was placed in the lower position. The retromammary dissection was performed using MCS, with counter-retracting using FBF and Maryland forceps. After full detachment of the breast parenchyma, the robotic docking was released to obtain the specimen. Because the incision size was too small to retrieve the specimen, the initial incision was extended to 3.7 cm (1 cm additional incision).
Right side mastectomy was initiated with a single 3.0 cm incision at the opposite side of the left breast. A blue dye was injected in the periareolar area for sentinel lymph node biopsy and along the border of the breast for the identification of the dissection boundary. The sentinel lymph node biopsy was performed through the axillary incision without the robotic system. After the sentinel lymph node biopsy, robotic mastectomy was performed in a similar manner as the left side. However, an intraoperative biopsy for the right nipple involvement of tumor cells was also performed. The docking time for the right side was 7 minutes. In brief, after the nipple areolar complex dissection, the nipple core was resected out to evaluate the involvement of tumor cells in a frozen section. An extra port was inserted into the GelPoint® and the nipple core was retrieved using the additional endoscopic forceps by the first assistant. The frozen section was negative for malignant cells. After completion of the dissection of the breast tissue, the initial incision was extended to 4.0 cm (1 cm additional incision) to retrieve the specimen. Immediate direct-to-implant reconstruction was performed by plastic surgeons without the use of the SP system. Acellular dermal matrix (ADM) was used to wrap the implant. The ADM was 16 × 16 cm in size and 1.5–2.3 cm in thickness. It was placed at the prepectoral pocket. Drains were inserted in the axilla and inframammary fold for both breasts.
The operation times were 170 minutes for the left breast and 180 minutes for the right. The console times were 93 minutes for the left breast and 109 minutes for the right. The total operation time including reconstruction was 619 minutes. The breast volumes were 194 g for the left breast and 192 g for the right.
There were no immediate postoperative complications, except for a minor skin burn on the right breast (
Figure 3). The patient was discharged from the hospital on post-operative day 15. The drain on the right side of the breast was removed on post-operative day 24 and the drain on the left side was removed on post-operative day 26. She re-visited the clinic on postoperative day 55 because of redness and heating sensation of the right breast. Antibiotics and conservative treatment including dressing and re-insertion of the drain were applied. Her symptoms subsided after 1 day of treatment and no major surgical intervention was needed.
Figure 3
Pre- and postoperative views of the patient. (A) Preoperative front view of the patient. (B) Postoperative front view of the patient 5 months postoperatively.

DISCUSSION
This study describes the first case of robotic mastectomy using the SP system. There were several advantages of the SP system. First, compared to multi-port systems consisting of the 3 separated robot arms and one camera, 4 instrument drives of the SP system were attached to one instrument arm. Therefore, the SP system enabled more detailed movement in the 2.5-cm narrow cannula and reduced the incision size. Second, gas-inflated robot-assisted mastectomy uses only 1 camera and 2 arms [
456]. Meanwhile, robotic mastectomy using the SP system can use the third arm with Cardiere or Maryland forceps which was particularly useful for the counter retraction of the tissue. However, this was different with multi-port systems. Third, the elbow movement of the robotic arms and articulation of the camera were other advantages of the SP system. In particular, the cobra position of the camera was useful for the visualization of the medial part of the breast. Furthermore, various camera modes and clutches enabled more detailed movements and visualization of the field. Surgeons can use the “relocate” mode to reposition the cannula (including the camera and robotic arms) more efficiently at the same time. The boom can rotate at least 360° using the port clutch, the instrument arm pitch moves to around 120°, and the instrument drives can rotate at least 360° [
78]. Another advantage of the SP system is that it has a smaller patient cart than previous versions.
However, there were some disadvantages of the SP system compared to the multi-port systems. The grasping power of the forceps in the SP system was lower compared to the multi-port system. For example, the most powerful forceps in the multi-port and SP systems were the ProGrasp and Cardiere forceps. The grasping force of the ProGrasp forceps was high whereas that of the Cardiere forceps was medium [
910]. For this reason, counter retraction during surgery in the SP system was weaker than that in the multi-port systems. Furthermore, the long elbow of the robot arms with weak grasping power in the SP system may interfere with effective counter retraction for breast tissue during surgery. Nonetheless, the lower grasping power did not significantly affect the performance of robot mastectomy using the SP system because the use of the third arm compensated for the weakness.
Even though there were some disadvantages with the SP system, the first case using the SP system was successfully performed without major postoperative complications. Further studies evaluating the difference between multi-port and SP systems are necessary to evaluate the usefulness of the updated robot surgical platform and data on the learning curve need to be generated in order to proceed with clinical validation.
ACKNOWLEDGMENTS
The cadaveric lab was supported by Intuitive Surgical, Sunnyvale, USA.
References
1. Toesca A, Peradze N, Galimberti V, Manconi A, Intra M, Gentilini O, et al. Robotic nipple-sparing mastectomy and immediate breast reconstruction with implant: first report of surgical technique. Ann Surg. 2017; 266:e28–e30.
2. Sarfati B, Honart JF, Leymarie N, Rimareix F, Al Khashnam H, Kolb F. Robotic da Vinci Xi-assisted nipple-sparing mastectomy: first clinical report. Breast J. 2018; 24:373–376.


4. Sarfati B, Struk S, Leymarie N, Honart JF, Alkhashnam H, Tran de Fremicourt K, et al. Robotic prophylactic nipple-sparing mastectomy with immediate prosthetic breast reconstruction: a prospective study. Ann Surg Oncol. 2018; 25:2579–2586.


5. Toesca A, Peradze N, Manconi A, Galimberti V, Intra M, Colleoni M, et al. Robotic nipple-sparing mastectomy for the treatment of breast cancer: feasibility and safety study. Breast. 2017; 31:51–56.

6. Lai HW, Chen ST, Lin SL, Chen CJ, Lin YL, Pai SH, et al. Robotic nipple-sparing mastectomy and immediate breast reconstruction with gel implant: technique, preliminary results and patient-reported cosmetic outcome. Ann Surg Oncol. 2019; 26:42–52.


8. Intuitive Surgical. Da Vinci SP Surgical System User Manual. Sunnyvale (CA): Intuitive Surgical;2018.
10. Intuitive Surgical. Da Vinci SP Surgical System Instrument & Accessory Catalog with Pricing in Korean Won. Sunnyvale (CA): Intuitive Surgical;2018.






 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download