Abstract
Objective
Materials and Methods
Results
Figures and Tables
 | Fig. 1Photograph of adjustable RF electrode (18 gauge, total length 8 cm).Simply sliding button enables operator to control length of exposed active tip. RF = radiofrequency
|
 | Fig. 2Schema of adjustable electrode procedure.Electrode is inserted by trans-isthmic approach. Smaller active tip (right) may be applied to smaller units in periphery of nodule, and larger active tip (left) may be used to ablate larger units in central portion of nodule, thereby completing RFA procedure with single electrode. RFA = radiofrequency ablation
|
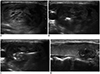 | Fig. 3RFA using adjustable electrode for benign thyroid nodule.
A. 33-year-old woman with thyroid nodule measuring 4.4 cm across largest diameter and 25.5 mL volume. Patient symptom and cosmetic scores were both 4. 1-cm active tip was applied to peripheral portion (B) and 1.5-cm active tip was applied to central portion (C) for treatment of this large-sized nodule. Since nodule was too large to treat in single session, medial section was left untreated (not shown). Additional ablation with fixed electrode was performed for remnant lesion. Six years later, follow-up ultrasound demonstrates that nodule (crosses) (D) has been reduced to 1 mL (volume reduction rate: 96.1%), and symptom and cosmetic scores were 0 and 1, respectively.
|
Table 3
Outcomes after RFA with Adjustable Electrodes

Values are presented as mean ± standard deviation. *Entire data of post RFA was calculated at 12-month follow-up or 6-month follow-up when follow-up period was less than 12 months, †Therapeutic success (volume reduction > 50%) at 12-month follow-up or 6-month follow-up when follow-up period was less than 12 months, ‡paired t test, §Wilcoxon signed rank test.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download