2. Brenner DJ, Hall EJ. Computed tomography — An increasing source of radiation exposure. N Engl J Med. 2007; 357:2277–2284.


4. Hara AK, Paden RG, Silva AC, Kujak JL, Lawder HJ, Pavlicek W. Iterative reconstruction technique for reducing body radiation dose at CT: feasibility study. AJR Am J Roentgenol. 2009; 193:764–771.


5. Nakayama Y, Awai K, Funama Y, Hatemura M, Imuta M, Nakaura T, et al. Abdominal CT with low tube voltage: preliminary observations about radiation dose, contrast enhancement, image quality, and noise. Radiology. 2005; 237:945–951.


6. Sagara Y, Hara AK, Pavlicek W, Silva AC, Paden RG, Wu Q. Abdominal CT: comparison of low-dose CT with adaptive statistical iterative reconstruction and routine-dose CT with filtered back projection in 53 patients. AJR Am J Roentgenol. 2010; 195:713–719.


7. Geyer LL, Schoepf UJ, Meinel FG, Nance JW Jr, Bastarrika G, Leipsic JA, et al. State of the art: iterative CT reconstruction techniques. Radiology. 2015; 276:339–357.


8. Holmquist F, Nyman U, Siemund R, Geijer M, Söderberg M. Impact of iterative reconstructions on image noise and low-contrast object detection in low kVp simulated abdominal CT: a phantom study. Acta Radiol. 2016; 57:1079–1088.


9. Prakash P, Kalra MK, Kambadakone AK, Pien H, Hsieh J, Blake MA, et al. Reducing abdominal CT radiation dose with adaptive statistical iterative reconstruction technique. Invest Radiol. 2010; 45:202–210.


11. Jain V, Seung S. Natural image denoising with convolutional networks. In : 23rd annual conference on neural information processing systems 22; 2009 December 7–10; Vancouver, Canada.
12. Nasri M, Nezamabadi-pour H. Image denoising in the wavelet domain using a new adaptive thresholding function. Neurocomputing. 2009; 72:1012–1025.

13. Xie J, Xu L, Chen E. Image denoising and inpainting with deep neural networks. In : 26th annual conference on neural information processing systems 25; 2012 December 3–6; Lake Tahoe, NV, USA.
14. Kang E, Min J, Ye JC. A deep convolutional neural network using directional wavelets for low-dose X-ray CT reconstruction. Med Phys. 2017; 44:e360–e375.

15. Chen H, Zhang Y, Zhang W, Liao P, Li K, Zhou J, et al. Low-dose CT via convolutional neural network. Biomed Opt Express. 2017; 8:679–694.


16. McCollough C. TU-FG-207A-04: overview of the low dose CT grand challenge. Med Phys. 2016; 43(Part 35):3759–3760.

17. Chen H, Zhang Y, Kalra MK, Lin F, Chen Y, Liao P, et al. Low-dose CT with a residual encoder-decoder convolutional neural network. IEEE Trans Med Imaging. 2017; 36:2524–2535.

18. Kang E, Chang W, Yoo J, Ye JC. Deep convolutional framelet denosing for low-dose CT via wavelet residual network. IEEE Trans Med Imaging. 2018; 37:1358–1369.


19. Ellmann S, Kammerer F, Brand M, Allmendinger T, May MS, Uder M, et al. A novel pairwise comparison-based method to determine radiation dose reduction potentials of iterative reconstruction algorithms, exemplified through circle of Willis computed tomography angiography. Invest Radiol. 2016; 51:331–339.

20. Ehman EC, Yu L, Manduca A, Hara AK, Shiung MM, Jondal D, et al. Methods for clinical evaluation of noise reduction techniques in abdominopelvic CT. Radiographics. 2014; 34:849–862.


21. Richard S, Husarik DB, Yadava G, Murphy SN, Samei E. Towards task-based assessment of CT performance: system and object MTF across different reconstruction algorithms. Med Phys. 2012; 39:4115–4122.


22. Friedman SN, Fung GS, Siewerdsen JH, Tsui BM. A simple approach to measure computed tomography (CT) modulation transfer function (MTF) and noise-power spectrum (NPS) using the American College of Radiology (ACR) accreditation phantom. Med Phys. 2013; 40:051907.

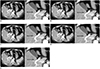
23. Kim K, Kim YH, Kim SY, Kim S, Lee YJ, Kim KP, et al. Low-dose abdominal CT for evaluating suspected appendicitis. N Engl J Med. 2012; 366:1596–1605.


24. Kim SY, Lee KH, Kim K, Kim TY, Lee HS, Hwang SS, et al. Acute appendicitis in young adults: low- versus standard-radiation-dose contrast-enhanced abdominal CT for diagnosis. Radiology. 2011; 260:437–445.


25. Christianson O, Chen JJ, Yang Z, Saiprasad G, Dima A, Filliben JJ, et al. An improved index of image quality for task-based performance of CT iterative reconstruction across three commercial implementations. Radiology. 2015; 275:725–734.









 PDF
PDF ePub
ePub Citation
Citation Print
Print







 XML Download
XML Download