INTRODUCTION
Cutaneous basal cell carcinoma (cBCC) is the most common histologic subtype of skin cancer, which is derived from the basal cells of the interfollicular epidermis and/or hair follicles. cBCCs most commonly occur during the seventh decade of life and affect men more frequently than women. The incidence rates of cBCC are related to the geographical latitude and are higher in lighter-skinned populations. Given the abundant evidence supporting the role of ultraviolet radiation exposure as the most important risk factor for cBCC carcinogenesis, cBCCs predominantly occur on the exposed parts of the body, with 75–85% of lesions found in the head and neck regions (
1234).
Cutaneous squamous cell carcinoma (cSCC) is the second most common type of skin cancer derived from epidermal keratinocytes exhibiting various degrees of differentiation. cSCCs predominantly occur during the eighth decade of life and are more common in men than women. Moreover, the highest incidence rates have been observed among white populations with fair complexion, blue eyes, and blonde or red hair. Considering that the risk of developing cSCC depends on the lifetime accumulation of ultraviolet radiation damage, predilection sites for cSCC include chronically exposed areas, such as the face (particularly the lips, ears, nose, cheeks, and eyelids) and dorsum of the hands (
45).
Although the metastasis rate of cBCC ranges from 0.0016% to 0.1343% (
6), approximately 1.9–4.9% of cSCCs metastasize to regional lymph nodes or more distant sites (
789). According to the National Comprehensive Cancer Network (NCCN) risk stratification, the recommended surgical margins for uninvolved skin around the tumor and/or biopsy site to a depth reaching mid-subcutaneous adipose tissue for standard excision was 4 mm in low-risk cBCCs (
10) and 4–6 mm in low-risk cSCCs (
11). In addition, for non-surgical candidates, the NCCN clinical practice guidelines recommend radiotherapy alone for cBCCs but combination chemoradiation therapy for cSCCs (
1011). Therefore, preoperative differentiation between cBCC and cSCC is essential for establishing appropriate decisions regarding the treatment strategy. Although a few studies have already described the imaging findings of cSCCs and cBCCs (
1213141516), to the best of our knowledge, no study has reported detailed magnetic resonance (MR) imaging findings that differentiate cBCCs from cSCCs. Hence, the present study aimed to assess MR imaging characteristics that can be used to differentiate cBCCs from cSCCs in the head and neck region.
MATERIALS AND METHODS
Patients
The study was approved by the human research committee of the Institutional Review Board of Gifu University (2019–126) and complied with the guidelines of the Health Insurance Portability and Accountability Act. The requirement for informed consent was waived due to the retrospective nature of this study. The electronic medical chart system of our University Hospital was searched for patients with histopathologically proven cBCCs and cSCCs in the head and neck region between January 2010 and December 2018. Among the identified patients, 21 with cBCCs and 16 with cSCCs underwent preoperative MR imaging, while seven with cBCCs and one with cSCCs were excluded given that they had a histological tumor height of < 4 mm, recurrent tumors, or inappropriate MR images. A total of 14 patients with primary cBCCs (eight men and six women; age range, 39–88 years; median age, 72 years) and 15 with primary cSCCs (11 men and four women; age range, 46–88 years; median age, 82 years) were included in this study.
MR Imaging
MR imaging was performed using a 1.5T MR imaging system (Intera Achieva 1.5T Pulsar; Philips Medical Systems, Best, The Netherlands) for seven cBCCs and seven cSCCs or a 3T MR imaging system (Intera Achieva 3.0T Quasar Dual; Philips Medical Systems) for seven cBCCs and eight cSCCs. MR imaging data were obtained using the parallel imaging technique at a section thickness of 3–4 mm with a 1-mm intersection gap, and a 16 × 16–20 × 20-cm field of view. Axial T1-weighted spin-echo (repetition time [TR]/echo time [TE], 387–752/9–16 ms), axial and oblique sagittal or coronal T2-weighted fast spin-echo (TR/TE, 3000–5982/90–120 ms), and axial and oblique sagittal or coronal fat-suppressed T2-weighted fast spin-echo (TR/TE, 3030–6123/80–120 ms) images were obtained for all 29 patients.
Imaging Data Analysis
Two radiologists with 20 and 6 years of post-training experience, respectively, in head and neck imaging independently reviewed all MR images, and any disagreements were resolved by consensus. The reviewers were unaware of the clinical findings and pathological diagnoses.
First, the reviewers noted the site of tumor occurrence, measured the maximum diameter and height of the lesion on T2-weighted images, and then calculated the diameter-to-height ratio.
Second, the reviewers evaluated the marginal characteristics of the tumors. They assessed the presence of superficial ulceration and protrusion into adjacent subcutaneous tissue on T2-weighted images. Deep tumor margins between the tumors and adjacent subcutaneous tissue were assessed on T2-weighted images and then classified into either ill-demarcated or well-demarcated. The presence of peritumoral fat stranding, which indicates increased signal intensity of the surrounding soft tissue, was assessed on fat-suppressed T2-weighted images.
Third, the reviewers evaluated the internal characteristics of the tumors. They assessed the presence and number of intratumoral T2-hyperintense foci, which were defined as well-demarcated, markedly hyperintense areas equivalent in intensity to the cerebrospinal fluid on fat-suppressed T2-weighted images. Intratumoral homogeneity was assessed using T2-weighted images, excluding intratumoral T2-hyperintense foci, and were classified as either homogeneous or heterogeneous.
Statistical Analysis
All statistical analyses were performed using SPSS version 22.0 (IBM Corp., Armonk, NY, USA). The chi-square test or Fisher's exact test was performed to compare the frequencies of qualitative assessments (occurrence site, superficial ulceration, protrusion into subcutaneous tissue, deep tumor margins, peritumoral fat stranding, intratumoral T2-hyperintense foci, and intratumoral homogeneity). The unpaired t test was used to compare the quantitative measurements (maximum diameter and height of the lesion, diameter-to-height ratio, and number of intratumoral T2-hyperintense foci). Interobserver variability of qualitative assessments was assessed using kappa statistics. P values < 0.05 were considered significant.
RESULTS
The qualitative imaging findings are summarized in
Table 1. cBCCs were located on the nose in 10 patients, eyelid in three, and auricle in one. Meanwhile, cSCCs were located on the cheek in five patients, scalp in three, nose in two, auricle in two, forehead in two, and mandible in one. cBCCs (71%) more frequently occurred on the nose than cSCCs (13%) (
p < 0.01).
The quantitative measurements are summarized in
Table 2. The maximum diameter (23.5 ± 7.2 mm vs. 12.7 ± 4.5 mm;
p < 0.01) and diameter-to-height ratio (2.8 ± 0.9 vs. 1.7 ± 0.4;
p < 0.01) were significantly greater in cSCCs than in cBCCs (
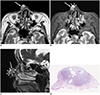
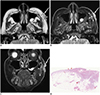
Figs. 1,
2). However, no significant difference in height (8.9 ± 3.5 mm vs. 7.7 ± 3.5 mm;
p = 0.208) was observed between cSCCs and cBCCs.
Superficial ulcer formation (67% vs. 21%;
p < 0.05), protrusion into subcutaneous tissue (60% vs. 21%;
p < 0.05), ill-demarcated deep tumor margins (60% vs. 7%;
p < 0.01), and peritumoral fat stranding (93% vs. 7%;
p < 0.01) were more frequently observed in cSCCs than in cBCCs (
Figs. 1,
2).
The intratumoral T2-hyperintense foci (57% vs. 13%;
p < 0.05) were more frequently observed in cBCCs than in cSCCs (
Figs. 1,
2). More than three intratumoral T2-hyperintense foci were observed in 6 of 14 cBCCs (43%), whereas they were not seen in cSCCs. However, no significant differences in the number of intratumoral T2-hyperintense foci (1.5 ± 0.7 vs. 8.9 ± 9.1;
p = 0.063) and intratumoral heterogeneous signal intensity (60% vs. 29%;
p = 0.089) were observed between cSCCs and cBCCs.
The kappa values for the two observers with regard to evaluating superficial ulcer formation, protrusion into subcutaneous tissue, ill-demarcated deep tumor margins, peritumoral fat stranding, and intratumoral heterogeneous signal intensity showed substantial agreement (0.72, 0.79, 0.64, 0.72, and 0.72, respectively), and that with regard to evaluating the intratumoral T2-hyperintense foci showed excellent agreement (1.00).
DISCUSSION
Predilection sites for cBCCs and cSCCs include the head and neck, given that such regions are more frequently exposed to ultraviolet radiation. The present study revealed that cBCCs (71%) were more frequently found on the nose than cSCCs (13%). Consistent with our results, other studies have also shown that the most common anatomical site for cBCCs was the nose (32–49%) (
217). In contrast, studies have also shown that cSCCs usually occur on the lateral aspect of the face, including the auricle, and auricular, buccal, parotid-masseteric, and temporal areas; therefore, only 5–14% of cSCCs occur on the nose (
1718). Differences in the distribution between cBCCs and cSCCs can thus be a clinically useful method for differentiating between both pathologies.
cBCCs have a variety of histopathological variants that always contain aggregates of basaloid cells with scant cytoplasm, as well as hyperchromatic nuclei surrounded by fibromyxoid stromal changes that induce retraction of the tumor from the stroma. Nodular cBCC, the most common variant, is characterized by large tumor nodules in the dermis, whereas infiltrating cBCC is characterized by narrow tumor cords and nests with an irregular, infiltrating growth pattern. Among the 14 cBCCs included in this study, 13 (93%) were nodular with well-demarcated deep tumor margins, whereas the remaining one (7%) infiltrated into the subcutaneous tissue with an ill-demarcated deep tumor margin and peritumoral fat stranding.
cSCCs usually progress from
in situ lesions to progressively more deeply invasive tumors.
In situ cSCCs may appear as roughened hyperkeratotic lesions mimicking benign keratosis, dermatoses, or lichen simplex chronicus. Well-differentiated cSCCs form crateriform lesions with central keratin plugs, whereas less well-differentiated cSCCs produce irregular, erythematous scaling nodules and plaques. cSCCs are considered invasive tumors, referring to cancer cells that have developed beyond the epidermis. Therefore, protrusion into the subcutaneous tissue (60%), ill-demarcated deep tumor margins (60%), and peritumoral fat stranding (93%) were frequently observed among the cSCCs included in this study. In addition, with invasion and progressive growth, hyperkeratotic nodules may develop ulceration. Malignant degeneration of untreated chronic wounds, referred to as Marjolin's ulcers, have been reported in cSCCs (
1920). Accordingly, superficial ulcer formation (67%) was also frequently observed in the cSCCs included in this study. These imaging findings correspond to the aggressive and invasive nature of cSCCs.
T2-weighted imaging has shown that cBCCs exhibit markedly higher hyperintensity relative to muscle and moderate hyperintensity relative to skin (
14). In the present study, intratumoral T2-hyperintense foci (57%) were frequently observed in cBCCs. A previous study evaluating ultrasonographic findings reported that hypersonographic spots in cBCCs seemed to correspond to calcification, horn cysts, or clusters of apoptotic cells at the centers of cBCC nests (
21). However, in all eight cBCCs with intratumoral T2-hyperintense foci, a radiologic-pathologic correlation on a one-to-one basis was performed and revealed cystic cavities filled with mucinous contents within cBCCs corresponding to the intratumoral T2-hyperintense foci, an MR imaging finding that may be characteristic to cBCCs.
The majority of cSCCs have a nonspecific signal intensity pattern of hypointensity on T1-weighted images and iso- to hyperintensity on T2-weighted images (
1319). However, as cSCCs progress and cystic necrosis develops within the tumor, they generate heterogeneous signal intensities, including non-enhanced regions, on contrast-enhanced images (
13). The cSCCs in this study frequently exhibited heterogeneous signal intensities on T2-weighted images (60%).
Several limitations of this study are worth noting. First, the cohort size was relatively small considering that the study was conducted at a single institution. Second, given that MR imaging could not accurately evaluate low-height tumors, only those with a histologic height of ≥ 4 mm were included in this study, which may have introduced a selection bias. Third, given that the cBCCs in this study were mostly nodular cBCCs (13/14), the features distinguishing cBCCs from cSCCs might not reflect characteristics of infiltrating cBCCs. Fourth, owing to the retrospective nature of this study, only five patients had undergone contrast-enhanced MR imaging. Among these five patients with tumors (three cBCCs and two cSCCs), relatively homogeneous enhancement was observed. In addition, peritumoral fat enhancement was observed only in tumors with peritumoral fat stranding on fat-suppressed T2-weighted images. Therefore, we presume that contrast-enhanced MR imaging has limited effectiveness for the differentiation between cBCCs and cSCCs. Fifth, according to NCCN guideline, imaging studies should be performed when extensive disease, such as bone involvement, perineural invasion, or deep soft tissue involvement, is suspected. As clinical diagnosis is routinely confirmed by biopsy, imaging-based differentiation is usually not required. This is the first study to reveal the differences in MR features between skin cancers; therefore, the present study may give provide recognition to the supplementary role of MR imaging in the differentiation of skin cancers.
In conclusion, cBCCs predominantly occurred on the nose with intratumoral T2-hyperintense foci corresponding to cystic cavities filled with mucinous contents within cBCCs, an MR imaging finding of intratumoral T2-hyperintense foci that may be characteristic. Meanwhile, characteristic MR imaging findings for cSCCs included a flattened configuration, superficial ulcer formation, protrusion into subcutaneous tissue, ill-demarcated deep tumor margins, and peritumoral fat stranding, which correspond to the aggressive and invasive nature of these tumors. We emphasize that this is the first study that compared the MR imaging findings of the most common skin cancers, in an era of growing prevalence of skin cancers, as well as an ageing population.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download