This article has been
cited by other articles in ScienceCentral.
Abstract
Objective
To evaluate the technical feasibility of intranodal lymphangiography and thoracic duct (TD) access in a canine model.
Materials and Methods
Five male mongrel dogs were studied. The dog was placed in the supine position, and the most prominent lymph node in the groin was accessed using a 26-gauge spinal needle under ultrasonography (US) guidance. If the cisterna chyli (CC) was not opacified by bilateral lymphangiography, the medial iliac lymph nodes were directly punctured and Lipiodol was injected. After opacification, the CC was directly punctured with a 22-gauge needle. A 0.018-in microguidewire was advanced through the CC and TD. A 4-Fr introducer and dilator were then advanced over the wire. The microguidewire was changed to a 0.035-in guidewire, and this was advanced into the left subclavian vein through the terminal valve of the TD. Retrograde TD access was performed using a snare kit.
Results
US-guided lymphangiography (including intranodal injection of Lipiodol [Guerbet]) was successful in all five dogs. However, in three of the five dogs (60%), the medial iliac lymph nodes were not fully opacified due to overt Lipiodol extravasation at the initial injection site. In these dogs, contralateral superficial inguinal intranodal injection was performed. However, two of these three dogs subsequently underwent direct medial iliac lymph node puncture under fluoroscopy guidance to deliver additional Lipiodol into the lymphatic system. Transabdominal CC puncture and cannulation with a 4-Fr introducer was successful in all five dogs. Transvenous retrograde catheterization of the TD (performed using a snare kit) was also successful in all five dogs.
Conclusion
A canine model may be appropriate for intranodal lymphangiography and TD access. Most lymphatic intervention techniques can be performed in a canine using the same instruments that are employed in a clinical setting.
Keywords: Lymphangiography, Lymphatic intervention, Canine, Thoracic duct, Cisterna chyli
INTRODUCTION
Lymphangiography and thoracic duct (TD) embolization are increasingly used in the treatment of chylothorax (
1234567). The clinical success rate of TD embolization has reached 97%, but its technical success rate remains relatively low. A recent meta-analysis reported a technical success rate of TD embolization of just 63.1% (95% confidence interval, 55.4–70.2%) (
8). The majority of technical failures are related to unsuccessful cannulation of the cisterna chyli (CC) or TD. Alternatively, percutaneous transvenous retrograde access can be performed (
910). However, this is technically challenging, especially if the operator does not have sufficient experience. Although TD embolization is a demanding procedure, requisite expertise is not always available.
We believe that prerequisite training using a large animal model would help to improve the outcome of human lymphatic intervention. Pigs and dogs have been suggested as large animal models because their lymphatic systems are anatomically similar to that of humans (
1112). The first percutaneous transabdominal TD catheterization was reported in a swine model (
13). More recently, pigs were found to have reproducible anatomy with multiple percutaneously accessible lymph nodes, making them suitable models for intranodal lymphangiography and subsequent TD access (
14). Although the use of lymphangiography techniques in canines has been reported in several studies (
1516), the feasibility of intranodal lymphangiography and subsequent TD access in a canine model has not been reported thus far. Therefore, the purpose of this study was to evaluate the technical feasibility of intranodal lymphangiography and TD access in a canine model.
MATERIALS AND METHODS
This study was approved by the Institutional Animal Care and Use Committee of University of Ulsan College of Medicine. Five male mongrel dogs weighing 40–50 kg were studied. The dogs were premedicated with acepromazine maleate (0.05 mg/kg, IM) and morphine (0.2 mg/kg, IM). Anesthesia was induced with thiopental (10 mg/kg, IV), and maintained with inhaled isoflurane (1–2%). All dogs were kept in a temperature-controlled room (24℃) with a fully automated 12-hour light-dark cycle. The dogs were supplied with autoclaved food and water.
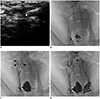
Before the procedure, general anesthesia was induced with an intramuscular injection of ketamine hydrochloride (Yuhan, Seoul, Korea) and atropine sulfate (Daewon, Seoul, Korea). General anesthesia was maintained with intravenous ketamine hydrochloride. The dog was placed in the supine position, and ultrasonography (US) of the right groin was performed to confirm the location of the superficial inguinal lymph nodes. The most prominent lymph node was accessed using a 26-gauge spinal needle (Hakko, Nagano, Japan) under US guidance (
Fig. 1). Lipiodol (Guerbet, Aulnay-Sous-Bois, France) was manually injected into the node at a rate of approximately 1 mL/5 min under fluoroscopic monitoring until the TD was visible (
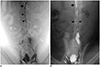
Fig. 2). A maximum of 10 cc of Lipiodol was used during this step. The injection site and thigh were gently massaged to expedite the flow of the Lipiodol. If the TD was not visible using unilateral access, the contralateral superficial inguinal lymph node was accessed, and Lipiodol was injected into this node. If the TD was still not visible, the medial iliac lymph nodes in the pelvic retroperitoneum were directly punctured using a 26-gauge spinal needle under fluoroscopy guidance to deliver additional Lipiodol into the lymphatic system.
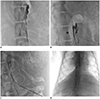
After opacification of the CC, a 22-gauge, 20-cm Chiba needle (Cook, Bloomington, IN, USA) was advanced into the CC (
Fig. 3). The location of the needle tip was verified using orthogonal projections. A 0.018-in guidewire (Transend, Boston Scientific, Fremont, CA, USA) was advanced through the needle into the CC, and subsequently into the TD. The needle was then removed, and a 4-Fr 10-cm outer catheter in introducer access set (Cook) were advanced over the 0.018-in guidewire under fluoroscopic guidance. With the coaxial introducer in place, lymphangiography of the TD was performed using Lipiodol. This provided roadmap images depicting the course of the upper TD and its venous insertion site. Following this, a 0.035-in, 180-cm hydrophilic guidewire (Terumo, Tokyo, Japan) was advanced into the TD through the terminal valve of the TD. The tip of the 0.035-in guidewire was located in the left subclavian vein.
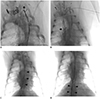
Next, the left brachial or axillary vein was accessed using a micropuncture kit (Cook) under US guidance. A 5-Fr sheath was placed into the vein, and a 4-Fr, 10-mm loop diameter Amplatz goose neck snare (Medtronic Inc., Minneapolis, MN, USA) was then introduced (
Fig. 4). The tip of the 0.035-in guidewire was captured, and was then retrieved externally using the goose neck snare. Following this, a 5-Fr, straight tip, 110-cm angiographic catheter (Soft-Vu, Angiodynamics Inc., Latham, NY, USA) was introduced into the TD over the 0.035-in guidewire. Lymphangiography of the TD was performed in a retrograde fashion using Lipiodol. Post-lymphangiographic non-contrast enhanced CT examinations (Sensation 16, Siemens Healthineers, Muenchen, Germany) were then performed (
Fig. 5). The CT scan parameters were as follows: collimation, 16 × 1.5 mm; pitch, 1.2; rotation time, 0.6 seconds; reference tube current–time products, 150 mAs at 100 kVp. After the CT scan, the dogs were euthanized by injecting an overdose of thiopental sodium.
RESULTS
The superficial inguinal lymph nodes were identified using US. The mean size of the largest lymph node of the group was 3.5 ± 1.0 cm on the longitudinal scan, and this node was located immediately lateral to the penis of the male dogs. Lymphangiography was successful in all five dogs and demonstrated drainage of the lymphatic collectors through the pelvic retroperitoneal wall to the medial iliac lymph nodes. However, in three of the five dogs (60%), the medial iliac lymph nodes were not fully opacified due to Lipiodol extravasation at the initial injection site. In these dogs, contralateral superficial inguinal intranodal injection was performed. However, two of these three dogs eventually required direct medial iliac lymph node puncture to facilitate opacification of the TD and CC.
Transabdominal CC puncture and cannulation with the 4-Fr introducer were successful in all five dogs. Transvenous retrograde catheterization of the TD using a snare kit was also successful in all five dogs. The final retrograde lymphangiography facilitated visualization of the whole TD. The shape and location of the terminal valve of the TD were found to be very similar to those of humans. In all five dogs, the post-lymphangiographic non-contrast enhanced CT images showed the entire course of the TD, the medial and superficial inguinal lymph nodes, and accumulated Lipiodol in the dependent portions of both lungs. The mean total procedure time was 92 ± 16 minutes.
DISCUSSION
TD embolization generally requires the following two steps: 1) contrast media is injected to fill and visualize the TD; and 2) the TD is cannulated by percutaneous puncture. TD cannulation can be technically challenging for a novice. Given that large animal experiments are expensive and infrequent, a robust animal model should allow for the same technical steps as those used in humans and have similar anatomy to humans. Understanding anatomical differences in the lymphatic system between animals is important for appropriate selection of a large animal model. Few studies have described anastomotic differences between species, likely due to a lack of effective techniques for comparison. However, because of recent developments in imaging technologies, the anatomy of the lymphatic system is better understood. Resultantly, recent studies have mapped lymphatic pathways, including superficial and deep lymph nodes, using fluorescence dye and radiopaque tracer. These tools allow us to compare anatomical similarities between species (
17).
The pig is considered a feasible animal model for lymphatic interventions (
1113). The first percutaneous transabdominal TD catheterization was reported by Cope in a swine model in 1995 (
13). Based on imaging finding, a recent study highlighted the suitability of the swine as a reproducible large animal model, given that it has four percutaneously accessible lymph node groups (
14). In swine, the superficial inguinal group drains superficially to the subiliac group. The subiliac group then follows a retroperitoneal course, and ultimately drains into the TD and CC. Unlike humans, swine do not have any relaying deep lymph node groups between the superficial lymph node groups and the TD. Furthermore, swine do not have superficial axillary lymph nodes; instead, the lymphatic vessels in their forelimbs are directly connected to the ventral cervical lymph nodes (
11).
However, the lymphatic system of canines is more anatomically similar to that of humans (
11). Furthermore, in canines, the superficial inguinal lymph nodes drain to the medial iliac lymph nodes before they reach the TD. This trajectory is similar to the lymphatic pathway in the human pelvis (
11). The medial iliac lymph node groups are found on both side walls of the pelvic retroperitoneum. Lipiodol should be continuously injected to opacify the medial iliac lymph nodes before lymph reaches the TD. If overt extravasation occurs at the injection site, Lipiodol is unable to fill the medial iliac lymph nodes. This extravasation leads to the failure of TD lymphangiography. Failure of the initial intranodal lymphangiography occurred in 60% of our canine models. Given the anatomical similarities between canines and humans, this phenomenon may also occur in humans. The described procedures appear to involve more elaborate lymphangiography techniques in humans and canines than in swine.
Various techniques can be practiced in a canine model. In addition to the elementary techniques of intranodal lymphangiography, a technique for direct pelvic lymph node puncture can be practiced only in a canine model. A safe course of puncture can be ensured with US guidance, and fluoroscopy guidance ensures appropriate targeting of the medial iliac lymph nodes. Once the medial iliac lymph nodes are punctured, Lipiodol can be directly injected into the retroperitoneal lymphatics, even after intranodal lymphangiography (via both superficial inguinal lymph nodes) failure.
Percutaneous transvenous retrograde access can be performed in cases in which the CC is inadequately opacified or absent (
910). However, the technical feasibility of retrograde access had not previously been investigated in an animal model. We successfully performed retrograde access, and confirmed that the shape and location of the terminal valve of the TD in canines are very similar to those of humans. Therefore, TD embolization or TD stenting by retrograde access can be practiced in a canine model before being performed in humans.
This study has several limitations. First, the technical feasibility of intranodal lymphangiography was not fully proven because only five mongrel dogs were studied. Second, anatomical variations of the lymphatic system was not encountered in this study. Third, further interventional procedures such as TD embolization or TD stenting have not been tested after TD cannulation. Fourth, although the lymphatic system of canines is anatomically similar to that of humans, it is not identical. In canines, the superficial inguinal lymph node is larger. Therefore, a longer injection time and greater injection volume may be required, which may lead to a high extravasation rate. Furthermore, canines have a thin layer of subcutaneous tissue, and this may be responsible for the high extravasation rate noted in this study.
In conclusion, our canine model was appropriate for intranodal lymphangiography and TD access. In this study, we found that the canine hind limb lymphatic anatomy is suitable for percutaneous access and experimental investigation. Therefore, most lymphatic techniques including lymphangiography, direct puncture of the pelvic lymph nodes and CC, and retrograde TD access can be performed in a canine using the same instruments that are employed in a clinical setting.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download