Abstract
Objective
Materials and Methods
Results
Figures and Tables
 | Fig. 1Correlation between three MTRs and histologic bowel fibrosis scores.Normalized MTR (r = 0.700; p < 0.001) (A) and standardized MTR (r = 0.695; p < 0.001) (B) were strongly correlated with bowel fibrosis scores, followed by MTR (r = 0.590; p < 0.001) (C). MTR = magnetization transfer ratio
|
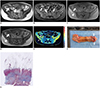 | Fig. 236-year-old woman with severe Crohn's disease in terminal ileum.
A. Axial T2-weighted image and (B) axial contrast-enhanced T1-weighted image show marked bowel wall thickening and luminal stenosis with hyper-enhancement at terminal ileum (arrows). C–E. Axial MT imaging without (C) and with (D) MT pulse as well as pseudo-color MTR map <(E) indicate that MT effect of terminal ileum (arrows; ROIs, 1–3; MTR, 44.2%; yellow-blue) is higher than that of normal small intestinal wall (hollow arrows; ROIs, 4–6; MTR, 26.0%; dark blue) and close to that of skeletal muscles (arrowheads; ROIs, 7–9; MTR, 52.0%; yellow). Averages of standardized MTR and normalized MTR of involved bowel wall are 0.70 and 0.85, respectively. F. Gross specimen from surgical excision demonstrates marked thickening of bowel wall with obvious luminal stenosis (arrows). G. Masson's trichrome staining (magnification: × 20) displays severe intestinal fibrosis (arrow; blue area) with fibrosis score of 3. MT = magnetization transfer, ROI = region of interest
|
 | Fig. 3Box plots showing differences of three MTRs between mild-to-moderate and severe bowel fibrosis.Significant differences in MTR (t = −4.470; p < 0.001) (A), normalized MTR (Z = −5.003; p < 0.001) (B), and standardized MTR (Z = −5.133; p < 0.001) (C) were found between mild-to-moderate and severe bowel fibrosis.
|
 | Fig. 4ROC curve analysis for differentiating severe bowel fibrosis from mild-to-moderate fibrosis.ROC curve analysis shows that standardized MTR is highly accurate with AUC of 0.895 in distinguishing severe intestinal fibrosis from mild-to-moderate intestinal fibrosis, followed by normalized MTR (AUC = 0.885) and MTR (AUC = 0.798). AUC = area under ROC curve, ROC = receiver operating characteristic
|




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download