INTRODUCTION
MATERIALS AND METHODS
Surgical procedures
Figure 1
(A) Use of a millimeter periodontal probe to determine the dimensions of the tissue in the donor area. (B) Flap containing epithelial tissue, connective tissue, and submucosal tissue being removed from the donor area. Care was taken to maintain a uniform thickness of the graft and to keep the periosteum as intact as possible. (C) Use of a No. 11 blade to separate the epithelial tissue from the connective tissue.

Figure 2
(A) Diamond bur used in the abrasion procedure. The flat structure aids in the removal of the epithelial tissue. (B) Removal of the epithelial tissue using a high-speed diamond-tip drill and abundant irrigation.
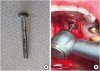
Figure 3
(A) SCTG removed. (B) SCTG, measuring 10 mm in length and 3 mm in width, with the 14 standardized measurements to be used in the histological analysis.
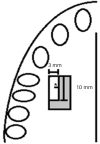
Figure 4
Schematic drawing showing the portion of the graft with the standardized measurements for use in the histological analysis. The section was obtained along the long axis of the graft with the region of choice facing the teeth of the patient. To identify the edge to be used for histology, a small incision (*) was performed on the opposite edge.
Histological analysis
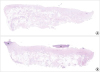
Figure 5
Histological image of a sample from group A analyzed in the Image Pro Plus 4.5 program, where tools were used to make measurements of the studied variables (3% of a ×20 lens).
1) The presence of an epithelial remnant, through direct visualization of present epithelial tissue.
2) Thickness of the epithelium, lamina propria, and submucosal tissue when present, through measurements at 5 points using the “grid mask” and “measurements” tools. The mean value of the 5 measurements was obtained for each of the tissue types.
3) The area of epithelium, lamina propria, and submucosal tissue present, through semi-automated segmentation in which the proportion of each tissue type was calculated in relation to the total area of the sample.
Statistical analysis
RESULTS
Presence of epithelial remnants
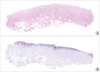
Figure 6
(A) Histological section of a sample from group B where no epithelial remnant was present (hematoxylin and eosin staining; 3% of a ×20 lens). (B) Histological section of a sample from group B where an epithelial remnant was present (*) (hematoxylin and eosin staining; 3% of a ×20 lens).
Area
Table 2
Composition of grafts





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download