METHODS
Patients
From May 1, 1991 to December 31, 2018, 43 patients underwent allogeneic HSCTs at the Departments of Pediatrics, Chonnam National University Hospital (CNUH) and Chonnam National University Hwasun Hospital (CNUHH). And their medical records were reviewed retrospectively. Baseline clinicopathologic data from patients, such as age, sex, initial blood counts, cellularity of BM, and etiology were recorded. Characteristics regarding to transplantation, such as, donor type, stem cell source, the degree of HLA matching, interval from diagnosis to transplantation, number of transfusions before transplantation, the presence of preceding IST, conditioning regimen, GvHD prophylaxis, engraftment, chimerism status, the presence of major viral infections, the presence of acute and chronic GvHD, transfusion-dependency, the need for second transplantation, and survival were analyzed.
Diagnostic criteria
All HSCTs were performed in patients with SAA or very severe aplastic anemia (VSAA). The diagnosis of SAA required at least two of the following criteria: an absolute neutrophil count (ANC) of less than 0.5 × 10
9/L, a platelet count (PLT) of less than 20 × 10
9/L, or a corrected reticulocyte count of less than 1% in the presence of hypocellular BM.
12 VSAA was reserved to patients when the neutrophil count was below 0.2 × 10
9/L.
1 Chromosome breakage test was performed to exclude congenital/inherited AA. Patients with clonal cytogenetic anomalies were excluded in the study population.
Conditioning regimen and GvHD prophylaxis
The conditioning regimen has been modified over time based on the donor type and the degree of HLA match. Initially, patients were conditioned with intravenous cyclophosphamide 50 mg/kg/d given for 4 consecutive days with horse ATG (Atgam; Pfizer, New York, NY, USA) 30 mg/kg/day for 3 days.
13 Additional procarbazine (12.5 mg/kg/day) was used in very early phase of the study. Since 2005, fludarabine (Flu)-based preparative regimen was used in most cases. The most prevalent preparative regimens in this study consisted of Flu 25 or 40 mg/m
2 and cyclophosphamide (Cy) 750 mg/m
2 for 4 days plus thoracoabdominal irradiation (TAI) 3 Gy the day before transplantation in matched related transplantation, and rabbit ATG (1.25 mg/kg/day for 4 days) was added in unrelated transplantation.
GvHD prophylaxis was continuous intravenous infusion of CsA (5 mg/kg/day) for matched related transplantation, or tacrolimus (0.03 mg/kg/day) plus short course of methotrexate (MTX) 15 mg/m2 at D+1, 10 mg/m2 at D+3, D+6, and D+11 for unrelated transplantation.
Supportive care
The patients were managed in a protective isolation room until the recovery of neutrophils. Immunoglobulin was administered every 3 weeks until 100 days after transplantation. Antifungals and pentamidine nebulizer were used to prevent infections. Donor chimerism was monitored at 1, 3, 6, 9, and 12 months after transplantation. Other routine measures of supportive care have been modified over the years. HLAs were initially serologically typed for Class I, but all HLA-A, -B, -C and -DRB1 were DNA typed since 2002.
Subgroup analyses
For patients with SAA or VSAA frontline HSCT was offered if there were MRDs. Salvage unrelated HSCTs were offered to the refractory/relapsed cases without MRDs after IST, while MRD transplants were performed in small number of cases of IST failure who initially presented with moderate diseases.
The patients were subdivided into subgroups by transplant strategies, mainly by conditioning regimens. The survival was evaluated as of December 31, 2018. Patients were censored at the time of the events or at time of last follow-up.
Outcome analyses
Complete response (CR) after IST or transplantation was defined as simultaneous fulfillment of the following: hemoglobin > 10 g/dL, ANC > 1.5 × 10
9/L and PLT > 100 × 10
9/L with evidence of normal hematopoiesis. Partial response (PR) was defined when the blood counts no longer met the diagnostic criteria of SAA, but still did not meet the criteria for CR. Patients who did not achieve CR or PR, or were transfusion-dependent at 3 months after transplantation were defined as non-responders.
13
OS was defined as the time between transplantation and death from any cause or time of last contact. FFS was calculated from the date of transplantation to last follow-up or first event (death due to any causes, lack of response, relapse, clinical paroxysmal nocturnal hemoglobinuria, secondary malignancy, HSCT after IST whichever occurred first).
7 GFFS was defined as the absence of grade 3–4 acute GvHD or chronic GvHD with systemic treatment in addition to the status of FFS.
9
Engraftment failure was defined by failure to achieve neutrophil engraftment, as evidenced by ANC < 0.5 × 10
9/L on D+28 post-transplant.
14 Graft failure after initial recovery of hematopoiesis was considered to be secondary graft failure. Donor-type aplasia was considered when patients had hypocellular BM with persistent cytopenia (hemoglobin < 8 g/L, neutrophils < 1.0 × 10
9/L, or platelets < 50 × 10
9/L) in full donor chimerism for > 6 months after achieving engraftment.
15
Major acute complications including acute GvHD, cytomegalovirus (CMV) reactivation and disease, Epstein-Barr virus (EBV)-lymphoproliferative disease, hepatic veno-occlusive disease (VOD), and hemorrhagic cystitis after transplantation were documented and their incidences were compared among subgroups.
Statistical analysis
Continuous variables were compared using the Student's t-test and categorical variables were compared using the χ2 test of Fisher's exact test. Probabilities of survival were estimated using the Kaplan-Meier (K-M) method and were compared using the log-rank test. Prognostic variables were evaluated by multivariable analyses using a Cox regression proportional hazard model. P value < 0.05 was considered statistically significant. The software package SPSS version 21.0 (SPSS Inc., Chicago, IL, USA) was used for all statistical analyses.
Ethics statement
This study was conducted as a retrospective, observational, descriptive study of clinical aspects of pediatric acquired AA, which was approved by the Institutional Review Board of the CNUHH (CNUHH-2019-118). Informed consent was obtained from legal guardians of the patients.
DISCUSSION
Over the past 4 decades, bone marrow transplantation (BMT) from a MRD has been the treatment of choice for children with acquired SAA. The treatment algorithm was based on the age, severity of AA, and the availability of MRD.
516 Recent studies in children have shown the OS rates exceeding 90% with BMT from MRD. IST using ATG and CsA has been considered as the first-line therapy for patients who lack a MRD, but those studies of IST also have shown comparable OS almost approaching 90%. However, FFS or event-free survival (EFS) after IST excluding deaths, lack of response, relapse, development of PNH, or secondary malignancy or HSCT showed only 3-year EFS of 33%, or 10-year FFS of 56%, respectively, which were significantly inferior to those after BMT from MRD.
7 In the retrospective, multicenter study of Korean Society of Pediatric Hematology-Oncology (KSPHO) from 1991 to 2005, the 10-year OS after BMT for MRD was 92.2%, which was comparable to other studies, but OS after alternative donor transplants, mostly after failed IST showed 47.1%,
11 which was less favorable than more recent studies, such as 78% OS after failed IST.
17
Second-line treatment is considered when the AA patient is refractory to frontline IST or is showing evidence of relapse after initial response. The relapse has been reported in up to 30% of responders to IST. As a second-line treatment, 2nd course of IST or matched unrelated donor (MUD) HSCT is considered depending on the availability of donor and age of the patient. Re-treatment with rabbit ATG and CsA showed reasonable response rate of 65% in relapsed cases but only 30% in refractory cases to initial horse ATG treatment.
18 Direct comparison of 2 second-line treatments, prospectively conducted by Japanese Society showed comparable 5-year OS (95.2% vs. 93.5%), but remarkable difference in FFS (9.4% vs. 83.9%;
P = 0.001) after 2nd IST (n = 21) or HSCT from alternative donor (n = 31), respectively.
19
The outcome of MUD transplantation has improved dramatically over the past two decades with better donor selection by DNA typing, better supportive care and experiences, showing comparable results with those of BMT from a MRD.
1920 Excellent survival of 95% at 5 years was reported after MUD HSCT post IST using FCC conditioning regimen (Flu, Cy [120–200 mg/kg], and alemtuzumab). The lower rate of acute and chronic GvHD in the study was partially related to a superior depletion of alloreactive donor T-cells with alemtuzumab as compared with ATG.
20 In a recent multicenter Korean study of MUD transplants a reduced-toxicity conditioning regimen (Flu, 200 mg/m
2; Cy, 120 mg/kg; and ATG, 7.5 mg/kg) showed excellent OS (96.7% vs. 67.9%;
P = 0.004) and EFS (93.3% vs. 64.3%;
P = 0.008, respectively) as compared to the previous conditioning regimen (Flu, 120 mg/m
2; Cy, 200 mg/kg; and ATG, 7.5 mg/kg).
21
Encouraged by those excellent MUD transplants comparable to MRD transplants, researchers employed upfront UD transplants in SAA children without prior IST. UK study showed excellent 2-year OS (96%) and 2-year EFS (92%) in upfront UD transplants (n = 29) after FCC conditioning, similar to 91% and 87%, respectively, after MRD transplants (n = 87). The OS and EFS after MUD HSCT post IST failure were inferior (74% each). The median interval from the diagnosis to HSCT was only 0.37 (0.15–1.3) years.
6 Another study from Korea showed excellent OS and EFS (91.3% each) after frontline alternative donor HSCT (n = 23), better than frontline IST group (n = 19) and salvage HSCT group (n = 11).
10 Thus, upfront MUD transplants should be pursued if suitable MUD, preferably 10/10 match, is available especially in the case of unavailability of horse ATG.
622
The development of acute and chronic GvHD has substantial effect on the quality of life of transplanted patients. Thus, GFFS, representing more ideal recovery after HSCT without ongoing morbidity, was recently introduced and evaluated in patients with hematologic malignancies and AA.
923 In a Swedish retrospective study of 68 adult AA patients the 5-year OS was 86.8%, but GFFS was 69.1%. Patients aged ≥ 40 years had a higher transplant-related mortality, and lower 5-year OS (70.6% vs. 92.2%;
P = 0.022), and a trend of lower GFFS (52.9% vs 74.5%;
P = 0.069).
24 A novel multicenter Chinese study of AA adults and children comparing upfront transplants showed excellent 1-year GFFS of 80.8% after haploidentical transplants, which was comparable to 88.4% after MRD transplants. Those results need to be reproduced in prospective studies to avoid selection bias.
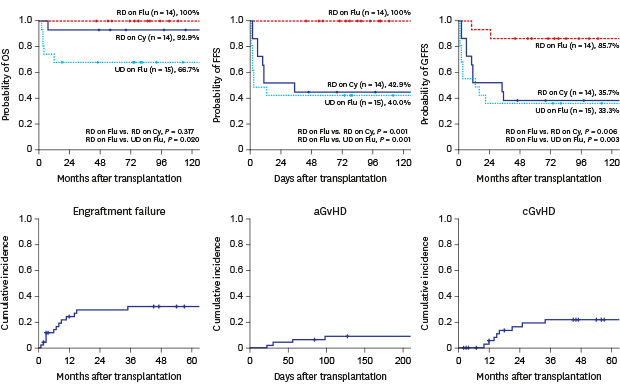
25 Thus, the current study is unique, real-world data evaluating GFFS in children with AA. GFFS was 51.2% in HSCT for 43 childhood AA patients, although OS was 86.0%. The best result of GFFS was seen in MSD transplantation with Flu-based conditioning (85.7%).
The standard conditioning regimen for MRD transplantation has been Cy (200 mg/kg) and ATG, showing excellent engraftment (95%) and long-term outcome (90% at 2 years) with reduced risk of GvHD.
26 However, due to high dose Cy-related toxicity, such as, cardiotoxicity, infertility, and hemorrhagic cystitis, transition of conditioning regimen from Cy/ATG to Flu, Cy plus ATG or alemtuzumab has been observed. The OS and FFS were above 90% in children after reduced intensity FCC conditioning.
20 Likewise, EBMT and British Society also recommend Flu-based conditioning regimen in adult cases.
1227 The addition of low dose total body irradiation (TBI) or TAI to the standard conditioning seems controversial, as that may have a role in preventing graft failure, but may increase transplant-related morbidity and mortality.
28
For the conditioning regimen in UD HSCT, the combination of Flu, Cy and ATG similar to those with MRD has been recommended from EBMT studies.
29 The dose of Cy was originally set at 300 mg/m
2 × 4, but the dose of Cy was increased to 120 mg/kg because of the significant risk of rejection. Low dose TBI, 2 Gy or 3 Gy has been added to reduce rejection following the American and Japanese studies.
3031 Furthermore, the addition of low dose TBI was regarded as one of the factors attributable to excellent OS after MUD HSCT, which was not statistically inferior to that of MRD transplants.
13 However, the beneficial effect should be weighed against the increased risk of second malignancies, infertility, growth failure and endocrine dysfunction. And another encouraging conditioning regimen is Flu (100–180 mg/m
2), melphalan (70–180 mg/m
2) and ATG by a Japanese Group. The OS and EFS were comparable to Flu/Cy-based conditioning.
32
In this study, there were considerable changes in transplantation techniques which included donor/recipient HLA matching, conditioning regimen, infection prophylaxis, and supportive care. Among them, various conditioning regimens have been used over time. However, Cy-based conditioning was substituted by Flu plus Cy in most cases since 2005. There was a discrepancy in survival rates. The higher OS of Cy-group was because all of them were MRD transplants (
Fig. 2A). But, Flu-based conditioning tended to have better FFS and GFFS than those with Cy-based conditioning, which might reflect better quality of life despite less favorable characteristics of Flu-group, such as less proportion (41.4%, 12/29;
P = 0.019) of MRDs and higher proportion of previous IST (62%, 18/29;
P = 0.012). These findings were in line with previous study showing less rejection with improved survival.
Graft failure has been a significant obstacle to a successful transplantation for SAA. The etiology is complex and very frequently multifactorial. Non-malignant diseases, such as AA seem to have higher incidence, but other factors including HLA mismatches, previous transfusion history, low CD34+ cell count, reduced intensity conditioning, graft source, viral infections, GvHD, drug toxicity, and others may be implicated. The cumulative incidence of graft failure was 15%–17% after MUD HSCT.
293031 The EBMT study reported the incidence of 17% after Flu, Cy and ATG with or without low dose TBI conditioning for alternative donor transplants after IST failure
29. Graft rejection was found in 10% after MSD and 8% after UD HSCT for AA patients.
33
The high incidence of graft failure (13/43; 30.2%), mostly with secondary graft failure (n = 12) in our study needs to be improved. The transition from Cy-based conditioning to Flu-based conditioning resulted in the incidence of secondary graft failure from 42.6% after Cy-based conditioning to 13.8% after Flu-based conditioning (
P = 0.035). Other measures including better HLA typing by DNA methods, better supportive care against viral infection and GvHD, shorter interval from diagnosis to HSCT with less transfusions, and incorporation of TBI in conditioning in most cases may have contributed to reduced incidence of graft failure in recent years. However, the presence of donor-specific anti-HLA antibodies (DSA) was not evaluated routinely in this study. Found in 10%–40% of cases, those antibodies are known to be associated with higher risk of graft rejection, especially in CB transplant and URD transplant settings.
34
Donor-type aplasia with poor graft function was seen in 23.3% (10/43) in our study. The incidence was decreased from 42.6% (6/14) after Cy-based conditioning to 13.8% (4/29) after Flu-based conditioning (
P = 0.035). On the other hand, a Japanese pediatric study (n = 660) showed the cumulative incidence of donor-type aplasia in 5.7% of AA cases with the predominance in Flu-based conditioning than in non-Flu group (11.6% vs. 2.7%;
P < 0.001).
35 Another Japanese study including AA patients (n = 26), and refractory cytopenia of childhood (RCC) patients (n = 24) showed the cumulative incidence of donor-type aplasia in 16% (8/50). It was found in 40% (6/15) after Flu/Cy conditioning regimen with low dose TBI, but in 6.5% (2/31) after Cy conditioning regimen with or without TBI (
P = 0.003). And it was more frequently seen in RCC patients (32%), which was found to be a significant risk factor (hazard ratio, 7.7;
P = 0.013) by multivariable analysis.
15 The high percentage of donor-type aplasia may need retrospective morphologic review to exclude the inclusion of RCC cases in this study.
The preferred stem cell source for all patients with AA including URD HSCT should be unmanipulated BM, as the use of PB stem cells is associated with an increased risk of chronic GvHD, and inferior outcome despite the earlier engraftment. Other factors found to positively affect OS after MUD HSCT were age ≤ 30 years, transplant within the first year after diagnosis, and CMV status.
13
As the presence of significant GvHD may jeopardize the quality of life, trials to decrease the incidence of GvHD have been attempted. ATG has been suggested to reduce high grade acute or chronic GvHD. In a recent meta-analysis, grade III/IV acute GvHD (risk ratio [RR] 0.52; 95% CI, 0.34–0.81;
P = 0.004), and chronic GvHD (RR, 0.52; 95% CI, 0.40–0.69;
P < 0.001) were decreased with the use of ATG.
36 However, the use of ATG was not significantly associated with reduced incidence of acute GvHD (
P = 0.388) and chronic GvHD (
P = 0.401), or improved survival including GFFS (
P = 0.726) in this study, probably because of small number and heterogeneity of our study. A retrospective study in the UK comparing ATG and alemtuzumab conditioning showed similar results but lower chronic GvHD in the alemtuzumab group (11% vs. 26%;
P = 0.031).
33 In the setting of MSD BMT, the use of rabbit ATG (8 mg/kg) in the conditioning showed protective effects against Grade II–IV acute GvHD, and moderate and severe chronic GvHD as compared with the use of horse ATG (90 mg/kg).
37
This study reviewed the outcomes of transplantation for pediatric AA along with changes of transplant strategies over last 25 years. The predominance of MRD transplants with Cy-based conditioning has evolved to increasing number of URD transplants, Flu-based conditioning, incorporation of irradiation, tacrolimus-based GvHD prophylaxis, and better supportive care. The FFS and GFFS tend to be higher in Flu-based conditioning group than in Cy-based group, especially in related transplantation, which may reflect better quality of life, although unfavorable factors were more frequent in the Flu-based group. Also, OS was quite comparable between 2 groups. However, systemic evaluation of quality of life by performance scales was not available in this retrospective study.
Graft failure including donor-type aplasia remains troublesome throughout this study. Change to Flu-based conditioning, incorporation of irradiation, the use of PB stem cells in some MUD transplants, all resulted in some reduction of graft failure, but the incidence is still high. Routine analysis of donor-specific anti-HLA antibodies, morphologic exclusion of RCC, better control of concomitant infections, and more effective GvHD control might be attempted to ensure stable, solid engraftment in patients with AA. Most importantly, either increase of Cy dose to 200 mg/kg, or change of conditioning regimen to Flu, Mel, ATG +/– irradiation should be considered to further decrease graft failure in our institution. These changes might be translated into improved FFS, and GFFS after transplant. Further efforts to minimize long-term complications, including radiation-related consequences should be pursued in the future.















 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download