1. Kanis JA. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: synopsis of a WHO report. WHO Study Group. Osteoporos Int. 1994; 4(6):368–381. PMID:
7696835.
2. Sozen T, Ozisik L, Basaran NC. An overview and management of osteoporosis. Eur J Rheumatol. 2017; 4(1):46–56. PMID:
28293453.

3. Kim KH, Lee K, Ko YJ, et al. Prevalence, awareness, and treatment of osteoporosis among Korean women: the Fourth Korea National Health and Nutrition Examination Survey. Bone. 2012; 50(5):1039–1047. PMID:
22366398.

4. Chalhoub D, Cawthon PM, Ensrud KE, et al. Risk of nonspine fractures in older adults with sarcopenia, low bone mass, or both. J Am Geriatr Soc. 2015; 63(9):1733–1740. PMID:
26310882.

5. Lee JK, Yoon BH, Oh CH, Kim JG, Han SH. Is sarcopenia a potential risk factor for distal radius fracture? Analysis using propensity score matching. J Bone Metab. 2018; 25(2):99–106. PMID:
29900159.

6. Patel HP, Syddall HE, Jameson K, et al. Prevalence of sarcopenia in community-dwelling older people in the UK using the European Working Group on Sarcopenia in Older People (EWGSOP) definition: findings from the Hertfordshire Cohort Study (HCS). Age Ageing. 2013; 42(3):378–384. PMID:
23384705.

7. Braithwaite RS, Col NF, Wong JB. Estimating hip fracture morbidity, mortality and costs. J Am Geriatr Soc. 2003; 51(3):364–370. PMID:
12588580.

8. Nellans KW, Kowalski E, Chung KC. The epidemiology of distal radius fractures. Hand Clin. 2012; 28(2):113–125. PMID:
22554654.

9. Bengner U, Johnell O. Increasing incidence of forearm fractures: a comparison of epidemiologic patterns 25 years apart. Acta Orthop Scand. 1985; 56(2):158–160. PMID:
4013706.
10. Melton LJ 3rd, Amadio PC, Crowson CS, O'Fallon WM. Long-term trends in the incidence of distal forearm fractures. Osteoporos Int. 1998; 8(4):341–348. PMID:
10024904.
11. Jo YH, Lee BG, Kim JH, et al. National surgical trends for distal radius fractures in Korea. J Korean Med Sci. 2017; 32(7):1181–1186. PMID:
28581277.

12. Roh YH, Koh YD, Noh JH, Gong HS, Baek GH. Evaluation of sarcopenia in patients with distal radius fractures. Arch Osteoporos. 2017; 12(1):5. PMID:
28004299.

13. Cho YJ, Gong HS, Song CH, Lee YH, Baek GH. Evaluation of physical performance level as a fall risk factor in women with a distal radial fracture. J Bone Joint Surg Am. 2014; 96(5):361–365. PMID:
24599196.

14. Rozental TD, Makhni EC, Day CS, Bouxsein ML. Improving evaluation and treatment for osteoporosis following distal radial fractures: a prospective randomized intervention. J Bone Joint Surg Am. 2008; 90(5):953–961. PMID:
18451385.
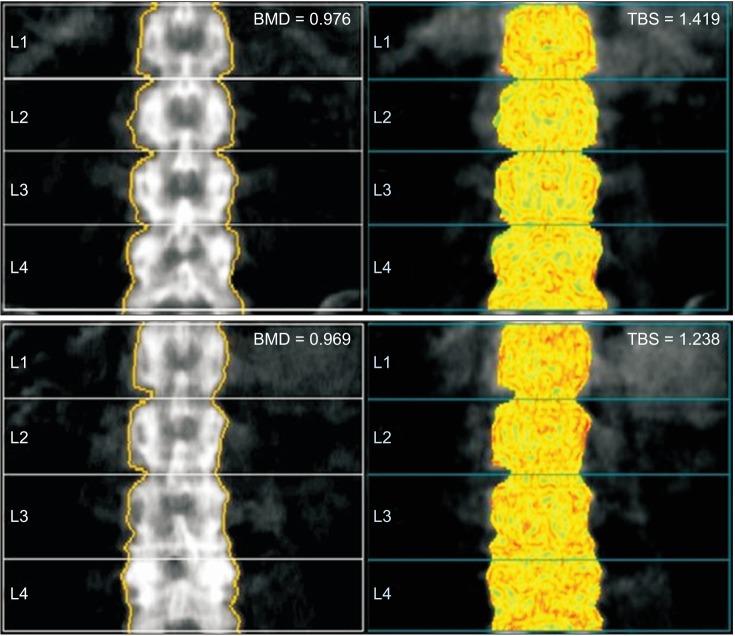
15. Shin YH, Gong HS, Gang DH, Shin HS, Kim J, Baek GH. Evaluation of trabecular bone score in patients with a distal radius fracture. Osteoporos Int. 2016; 27(12):3559–3565. PMID:
27341808.

16. Kanis JA, McCloskey EV, Johansson H, Oden A, Melton LJ 3rd, Khaltaev N. A reference standard for the description of osteoporosis. Bone. 2008; 42(3):467–475. PMID:
18180210.

17. Itoh S, Ohta T, Samejima H, Shinomiya K. Bone mineral density in the distal radius in a healthy Japanese population and in relation to fractures of the distal radius. J Hand Surg Br. 1999; 24(3):334–337. PMID:
10433449.

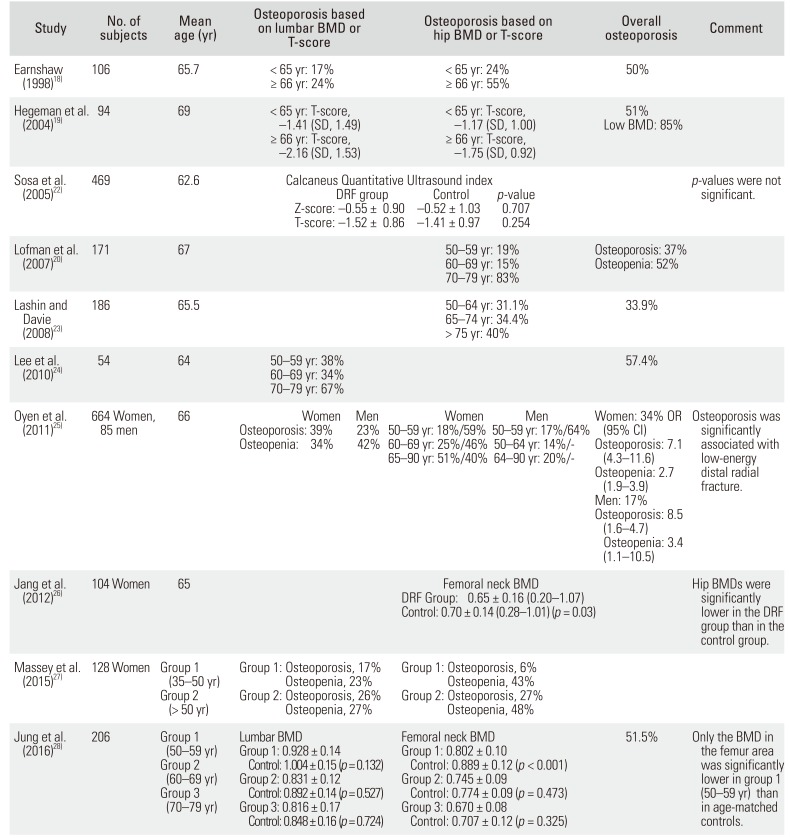
18. Earnshaw SA, Cawte SA, Worley A, Hosking DJ. Colles' fracture of the wrist as an indicator of underlying osteoporosis in postmenopausal women: a prospective study of bone mineral density and bone turnover rate. Osteoporos Int. 1998; 8(1):53–60. PMID:
9692078.

19. Hegeman JH, Oskam J, van der Palen J, Ten Duis HJ, Vierhout PA. The distal radial fracture in elderly women and the bone mineral density of the lumbar spine and hip. J Hand Surg Br. 2004; 29(5):473–476. PMID:
15336753.

20. Lofman O, Hallberg I, Berglund K, et al. Women with low-energy fracture should be investigated for osteoporosis. Acta Orthop. 2007; 78(6):813–821. PMID:
18236189.
21. Nordvall H, Glanberg-Persson G, Lysholm J. Are distal radius fractures due to fragility or to falls? A consecutive case-control study of bone mineral density, tendency to fall, risk factors for osteoporosis, and health-related quality of life. Acta Orthop. 2007; 78(2):271–277. PMID:
17464618.

22. Sosa M, Saavedra P, Gomez-Alonso C, et al. Postmenopausal women with Colles' fracture have bone mineral density values similar to those of controls when measured with calcaneus quantitative ultrasound. Eur J Intern Med. 2005; 16(8):561–566. PMID:
16314236.

23. Lashin H, Davie MW. DXA scanning in women over 50 years with distal forearm fracture shows osteoporosis is infrequent until age 65 years. Int J Clin Pract. 2008; 62(3):388–393. PMID:
17535301.
24. Lee JO, Chung MS, Baek GH, Oh JH, Lee YH, Gong HS. Age- and site-related bone mineral densities in Korean women with a distal radius fracture compared with the reference Korean female population. J Hand Surg Am. 2010; 35(9):1435–1441. PMID:
20807621.

25. Oyen J, Brudvik C, Gjesdal CG, Tell GS, Lie SA, Hove LM. Osteoporosis as a risk factor for distal radial fractures: a case-control study. J Bone Joint Surg Am. 2011; 93(4):348–356. PMID:
21325586.
26. Jang WY, Chung MS, Baek GH, Song CH, Cho HE, Gong HS. Vitamin D levels in post-menopausal Korean women with a distal radius fracture. Injury. 2012; 43(2):237–241. PMID:
22088327.

27. Massey PA, James JR, Bonvillain J, Nelson BG, Massey SR, Hollister A. Prevalence of low bone mineral density in younger versus older women with distal radius fractures. Am J Orthop (Belle Mead NJ). 2015; 44(12):E493–E496. PMID:
26665250.
28. Jung HJ, Park HY, Kim JS, Yoon JO, Jeon IH. Bone mineral density and prevalence of osteoporosis in postmenopausal Korean women with low-energy distal radius fractures. J Korean Med Sci. 2016; 31(6):972–975. PMID:
27247508.

29. Stone KL, Seeley DG, Lui LY, et al. BMD at multiple sites and risk of fracture of multiple types: long-term results from the Study of Osteoporotic Fractures. J Bone Miner Res. 2003; 18(11):1947–1954. PMID:
14606506.

30. Rubin CD. Emerging concepts in osteoporosis and bone strength. Curr Med Res Opin. 2005; 21(7):1049–1056. PMID:
16004672.

31. Silva BC, Leslie WD, Resch H, et al. Trabecular bone score: a noninvasive analytical method based upon the DXA image. J Bone Miner Res. 2014; 29(3):518–530. PMID:
24443324.

32. Briot K, Paternotte S, Kolta S, et al. Added value of trabecular bone score to bone mineral density for prediction of osteoporotic fractures in postmenopausal women: the OPUS study. Bone. 2013; 57(1):232–236. PMID:
23948677.

33. Krueger D, Fidler E, Libber J, Aubry-Rozier B, Hans D, Binkley N. Spine trabecular bone score subsequent to bone mineral density improves fracture discrimination in women. J Clin Densitom. 2014; 17(1):60–65. PMID:
23769698.

34. Mackey DC, Eby JG, Harris F, et al. Prediction of clinical non-spine fractures in older black and white men and women with volumetric BMD of the spine and areal BMD of the hip: the Health, Aging, and Body Composition Study. J Bone Miner Res. 2007; 22(12):1862–1868. PMID:
17708713.

35. Manhard MK, Nyman JS, Does MD. Advances in imaging approaches to fracture risk evaluation. Transl Res. 2017; 181:1–14. PMID:
27816505.

36. Schultz K, Wolf JM. Emerging technologies in osteoporosis diagnosis. J Hand Surg Am. 2019; 44(3):240–243. PMID:
30177358.
37. Schreiber JJ, Anderson PA, Rosas HG, Buchholz AL, Au AG. Hounsfield units for assessing bone mineral density and strength: a tool for osteoporosis management. J Bone Joint Surg Am. 2011; 93(11):1057–1063. PMID:
21655899.

38. Johnson CC, Gausden EB, Weiland AJ, Lane JM, Schreiber JJ. Using Hounsfield units to assess osteoporotic status on wrist computed tomography scans: comparison with dual energy x-ray absorptiometry. J Hand Surg Am. 2016; 41(7):767–774. PMID:
27189150.

39. Link TM. Osteoporosis imaging: state of the art and advanced imaging. Radiology. 2012; 263(1):3–17. PMID:
22438439.

40. Gausden EB, Nwachukwu BU, Schreiber JJ, Lorich DG, Lane JM. Opportunistic use of CT imaging for osteoporosis screening and bone density assessment: a qualitative systematic review. J Bone Joint Surg Am. 2017; 99(18):1580–1590. PMID:
28926388.
41. MacNeil JA, Boyd SK. Accuracy of high-resolution peripheral quantitative computed tomography for measurement of bone quality. Med Eng Phys. 2007; 29(10):1096–1105. PMID:
17229586.

42. Burghardt AJ, Kazakia GJ, Majumdar S. A local adaptive threshold strategy for high resolution peripheral quantitative computed tomography of trabecular bone. Ann Biomed Eng. 2007; 35(10):1678–1686. PMID:
17602299.

43. Macneil JA, Boyd SK. Bone strength at the distal radius can be estimated from high-resolution peripheral quantitative computed tomography and the finite element method. Bone. 2008; 42(6):1203–1213. PMID:
18358799.

44. Aaron JE, Francis RM, Peacock M, Makins NB. Contrasting microanatomy of idiopathic and corticosteroid-induced osteoporosis. Clin Orthop Relat Res. 1989; (243):294–305.

45. Bala Y, Zebaze R, Seeman E. Role of cortical bone in bone fragility. Curr Opin Rheumatol. 2015; 27(4):406–413. PMID:
26002033.

46. Burr D. Microdamage and bone strength. Osteoporos Int. 2003; 14 Suppl 5:S67–S72. PMID:
14504709.

47. Bala Y, Zebaze R, Ghasem-Zadeh A, et al. Cortical porosity identifies women with osteopenia at increased risk for forearm fractures. J Bone Miner Res. 2014; 29(6):1356–1362. PMID:
24519558.

48. Biver E, Durosier-Izart C, Chevalley T, van Rietbergen B, Rizzoli R, Ferrari S. Evaluation of radius microstructure and areal bone mineral density improves fracture prediction in postmenopausal women. J Bone Miner Res. 2018; 33(2):328–337. PMID:
28960489.

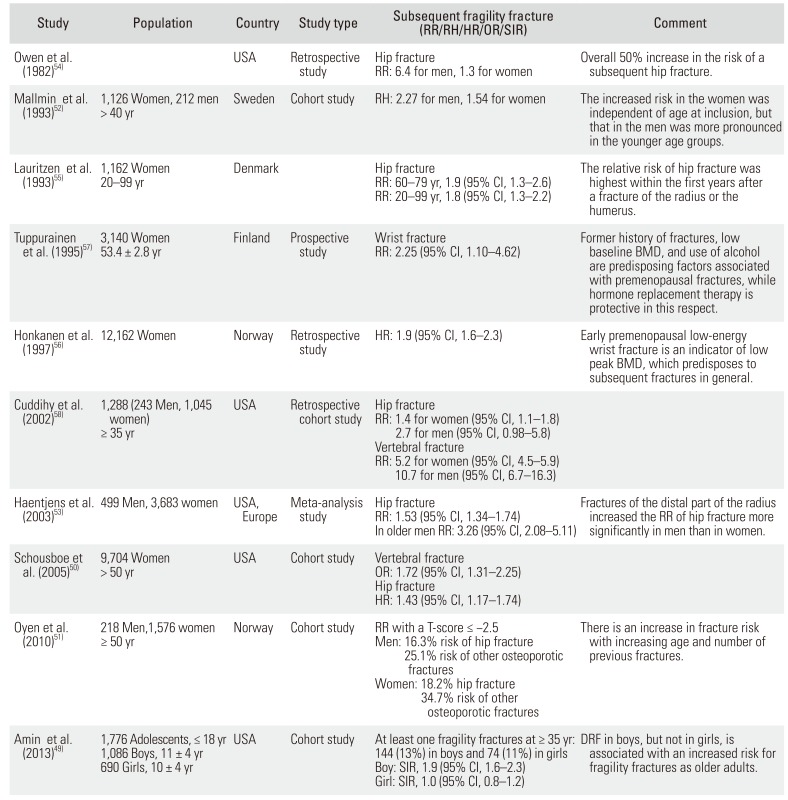
49. Amin S, Melton LJ 3rd, Achenbach SJ, et al. A distal forearm fracture in childhood is associated with an increased risk for future fragility fractures in adult men, but not women. J Bone Miner Res. 2013; 28(8):1751–1759. PMID:
23456800.

50. Schousboe JT, Fink HA, Taylor BC, et al. Association between self-reported prior wrist fractures and risk of subsequent hip and radiographic vertebral fractures in older women: a prospective study. J Bone Miner Res. 2005; 20(1):100–106. PMID:
15619675.

51. Oyen J, Gjesdal CG, Brudvik C, et al. Low-energy distal radius fractures in middle-aged and elderly men and women: the burden of osteoporosis and fracture risk: a study of 1794 consecutive patients. Osteoporos Int. 2010; 21(7):1257–1267. PMID:
19813045.
52. Mallmin H, Ljunghall S, Persson I, Naessen T, Krusemo UB, Bergstrom R. Fracture of the distal forearm as a forecaster of subsequent hip fracture: a population-based cohort study with 24 years of follow-up. Calcif Tissue Int. 1993; 52(4):269–272. PMID:
8467406.

53. Haentjens P, Autier P, Collins J, Velkeniers B, Vanderschueren D, Boonen S. Colles fracture, spine fracture, and subsequent risk of hip fracture in men and women: a meta-analysis. J Bone Joint Surg Am. 2003; 85(10):1936–1943. PMID:
14563801.
54. Owen RA, Melton LJ 3rd, Ilstrup DM, Johnson KA, Riggs BL. Colles' fracture and subsequent hip fracture risk. Clin Orthop Relat Res. 1982; (171):37–43.

55. Lauritzen JB, Schwarz P, McNair P, Lund B, Transbol I. Radial and humeral fractures as predictors of subsequent hip, radial or humeral fractures in women, and their seasonal variation. Osteoporos Int. 1993; 3(3):133–137. PMID:
8481589.

56. Honkanen R, Tuppurainen M, Kroger H, Alhava E, Puntila E. Associations of early premenopausal fractures with subsequent fractures vary by sites and mechanisms of fractures. Calcif Tissue Int. 1997; 60(4):327–331. PMID:
9075627.

57. Tuppurainen M, Kroger H, Honkanen R, et al. Risks of perimenopausal fractures: a prospective population-based study. Acta Obstet Gynecol Scand. 1995; 74(8):624–628. PMID:
7660769.
58. Cuddihy MT, Gabriel SE, Crowson CS, et al. Osteoporosis intervention following distal forearm fractures: a missed opportunity? Arch Intern Med. 2002; 162(4):421–426. PMID:
11863474.
59. Gregory JS, Aspden RM. Femoral geometry as a risk factor for osteoporotic hip fracture in men and women. Med Eng Phys. 2008; 30(10):1275–1286. PMID:
18976949.

60. Shin YH, Gong HS, Kim KM, Lee JH, Kwon O, Baek GH. Evaluation of hip geometry parameters in patients with a distal radius fracture. J Clin Densitom. 2019.

61. Melton LJ 3rd, Riggs BL, Keaveny TM, et al. Structural determinants of vertebral fracture risk. J Bone Miner Res. 2007; 22(12):1885–1892. PMID:
17680721.

62. McQueen MM, Hajducka C, Court-Brown CM. Redisplaced unstable fractures of the distal radius: a prospective randomised comparison of four methods of treatment. J Bone Joint Surg Br. 1996; 78(3):404–409. PMID:
8636175.
63. Itoh S, Tomioka H, Tanaka J, Shinomiya K. Relationship between bone mineral density of the distal radius and ulna and fracture characteristics. J Hand Surg Am. 2004; 29(1):123–130. PMID:
14751115.

64. Greatting MD, Bishop AT. Intrafocal (Kapandji) pinning of unstable fractures of the distal radius. Orthop Clin North Am. 1993; 24(2):301–307. PMID:
8479727.

65. Arora R, Gabl M, Erhart S, Schmidle G, Dallapozza C, Lutz M. Aspects of current management of distal radius fractures in the elderly individuals. Geriatr Orthop Surg Rehabil. 2011; 2(5-6):187–194. PMID:
23569689.

66. Chung KC, Petruska EA. Treatment of unstable distal radial fractures with the volar locking plating system: surgical technique. J Bone Joint Surg Am. 2007; 89 Suppl 2 Pt.2:256–266. PMID:
17768220.
67. Nana AD, Joshi A, Lichtman DM. Plating of the distal radius. J Am Acad Orthop Surg. 2005; 13(3):159–171. PMID:
15938605.

68. Lee JI, Park KC, Joo IH, Jeong HW, Park JW. The effect of osteoporosis on the outcomes after volar locking plate fixation in female patients older than 50 years with unstable distal radius fractures. J Hand Surg Am. 2018; 43(8):731–737. PMID:
30042026.

69. Saving J, Severin Wahlgren S, Olsson K, et al. Nonoperative treatment compared with volar locking plate fixation for dorsally displaced distal radial fractures in the elderly: a randomized controlled trial. J Bone Joint Surg Am. 2019; 101(11):961–969. PMID:
31169572.
70. Buyukkurt CD, Bulbul M, Ayanoglu S, Esenyel CZ, Ozturk K, Gurbuz H. The effects of osteoporosis on functional outcome in patients with distal radius fracture treated with plate osteosynthesis. Acta Orthop Traumatol Turc. 2012; 46(2):89–95. PMID:
22491432.

71. Figl M, Weninger P, Jurkowitsch J, Hofbauer M, Schauer J, Leixnering M. Unstable distal radius fractures in the elderly patient: volar fixed-angle plate osteosynthesis prevents secondary loss of reduction. J Trauma. 2010; 68(4):992–998. PMID:
20065876.
72. Rhee SH, Kim J, Lee YH, Gong HS, Lee HJ, Baek GH. Factors affecting late displacement following volar locking plate fixation for distal radial fractures in elderly female patients. Bone Joint J. 2013; 95(3):396–400. PMID:
23450027.

73. Fitzpatrick SK, Casemyr NE, Zurakowski D, Day CS, Rozental TD. The effect of osteoporosis on outcomes of operatively treated distal radius fractures. J Hand Surg Am. 2012; 37(10):2027–2034. PMID:
22938805.

74. Roh YH, Lee BK, Noh JH, Oh JH, Gong HS, Baek GH. Factors delaying recovery after volar plate fixation of distal radius fractures. J Hand Surg Am. 2014; 39(8):1465–1470. PMID:
24908556.

75. Choi WS, Lee HJ, Kim DY, et al. Does osteoporosis have a negative effect on the functional outcome of an osteoporotic distal radial fracture treated with a volar locking plate? Bone Joint J. 2015; 97(2):229–234. PMID:
25628287.

76. Voigt C, Plesz A, Jensen G, Katthagen C, Lill H. Volar locking plating for distal radial fractures: is osteoporosis associated with poorer functional results and higher complications rates? Chirurg. 2012; 83(5):463–471. PMID:
21866388.
77. Xu SW, Yu R, Zhao GF, Wang JW. Early period of fracture healing in ovariectomized rats. Chin J Traumatol. 2003; 6(3):160–166. PMID:
12749788.
78. Axelrod TS, McMurtry RY. Open reduction and internal fixation of comminuted, intraarticular fractures of the distal radius. J Hand Surg Am. 1990; 15(1):1–11. PMID:
2299146.

79. Ladd AL, Pliam NB. The role of bone graft and alternatives in unstable distal radius fracture treatment. Orthop Clin North Am. 2001; 32(2):337–351. ixPMID:
11331546.

80. Cosman F, de Beur SJ, LeBoff MS, et al. Clinician's guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014; 25(10):2359–2381. PMID:
25182228.

81. Russow G, Jahn D, Appelt J, Mardian S, Tsitsilonis S, Keller J. Anabolic therapies in osteoporosis and bone regeneration. Int J Mol Sci. 2018; 20(1):E83. PMID:
30587780.

82. Gong HS, Song CH, Lee YH, Rhee SH, Lee HJ, Baek GH. Early initiation of bisphosphonate does not affect healing and outcomes of volar plate fixation of osteoporotic distal radial fractures. J Bone Joint Surg Am. 2012; 94(19):1729–1736. PMID:
22992762.

83. Adami S, Libanati C, Boonen S, et al. Denosumab treatment in postmenopausal women with osteoporosis does not interfere with fracture-healing: results from the FREEDOM trial. J Bone Joint Surg Am. 2012; 94(23):2113–2119. PMID:
23097066.
84. Aspenberg P, Genant HK, Johansson T, et al. Teriparatide for acceleration of fracture repair in humans: a prospective, randomized, double-blind study of 102 postmenopausal women with distal radial fractures. J Bone Miner Res. 2010; 25(2):404–414. PMID:
19594305.

85. Saag KG, Petersen J, Brandi ML, et al. Romosozumab or alendronate for fracture prevention in women with osteoporosis. N Engl J Med. 2017; 377(15):1417–1427. PMID:
28892457.

86. Bandeira L, Lewiecki EM, Bilezikian JP. Romosozumab for the treatment of osteoporosis. Expert Opin Biol Ther. 2017; 17(2):255–263. PMID:
28064540.

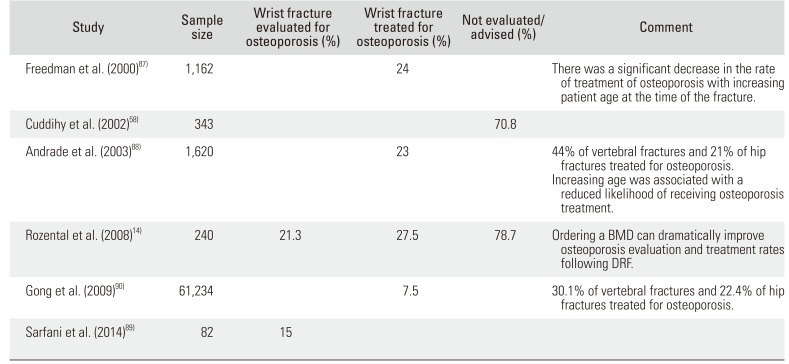
87. Freedman KB, Kaplan FS, Bilker WB, Strom BL, Lowe RA. Treatment of osteoporosis: are physicians missing an opportunity? J Bone Joint Surg Am. 2000; 82(8):1063–1070. PMID:
10954094.

88. Andrade SE, Majumdar SR, Chan KA, et al. Low frequency of treatment of osteoporosis among postmenopausal women following a fracture. Arch Intern Med. 2003; 163(17):2052–2057. PMID:
14504118.

89. Sarfani S, Scrabeck T, Kearns AE, Berger RA, Kakar S. Clinical efficacy of a fragility care program in distal radius fracture patients. J Hand Surg Am. 2014; 39(4):664–669. PMID:
24576753.

90. Gong HS, Oh WS, Chung MS, Oh JH, Lee YH, Baek GH. Patients with wrist fractures are less likely to be evaluated and managed for osteoporosis. J Bone Joint Surg Am. 2009; 91(10):2376–2380. PMID:
19797572.

91. Roh YH, Noh JH, Gong HS, Baek GH. Comparative adherence to weekly oral and quarterly intravenous bisphosphonates among patients with limited heath literacy who sustained distal radius fractures. J Bone Miner Metab. 2018; 36(5):589–595. PMID:
28983705.

92. Roh YH, Lee ES, Ahn J, et al. Factors affecting willingness to get assessed and treated for osteoporosis. Osteoporos Int. 2019; 30(7):1395–1401. PMID:
30944954.

93. Curtis JR, Silverman SL. Commentary: the five Ws of a Fracture Liaison Service. Why, who, what, where, and how? In osteoporosis, we reap what we sow. Curr Osteoporos Rep. 2013; 11(4):365–368. PMID:
24104520.

94. Kaufman JD, Bolander ME, Bunta AD, Edwards BJ, Fitzpatrick LA, Simonelli C. Barriers and solutions to osteoporosis care in patients with a hip fracture. J Bone Joint Surg Am. 2003; 85(9):1837–1843. PMID:
12954849.

95. Akesson K, Marsh D, Mitchell PJ, et al. Capture the Fracture: a Best Practice Framework and global campaign to break the fragility fracture cycle. Osteoporos Int. 2013; 24(8):2135–2152. PMID:
23589162.

96. Sujic R, Beaton DE, Mamdani M, et al. Five-year refracture rates of a province-wide fracture liaison service. Osteoporos Int. 2019; 30(8):1671–1677. PMID:
31152183.

97. Santilli V, Bernetti A, Mangone M, Paoloni M. Clinical definition of sarcopenia. Clin Cases Miner Bone Metab. 2014; 11(3):177–180. PMID:
25568649.

98. He H, Liu Y, Tian Q, Papasian CJ, Hu T, Deng HW. Relationship of sarcopenia and body composition with osteoporosis. Osteoporos Int. 2016; 27(2):473–482. PMID:
26243357.

99. Benichou O, Lord SR. Rationale for strengthening muscle to prevent falls and fractures: a review of the evidence. Calcif Tissue Int. 2016; 98(6):531–545. PMID:
26847435.

100. Fujita K, Kaburagi H, Nimura A, et al. Lower grip strength and dynamic body balance in women with distal radial fractures. Osteoporos Int. 2019; 30(5):949–956. PMID:
30607458.

101. Roh YH, Noh JH, Gong HS, Baek GH. Effect of low appendicular lean mass, grip strength, and gait speed on the functional outcome after surgery for distal radius fractures. Arch Osteoporos. 2017; 12(1):41. PMID:
28411349.

102. Reid IR. Vitamin D effect on bone mineral density and fractures. Endocrinol Metab Clin North Am. 2017; 46(4):935–945. PMID:
29080644.

103. Oyen J, Apalset EM, Gjesdal CG, Brudvik C, Lie SA, Hove LM. Vitamin D inadequacy is associated with low-energy distal radius fractures: a case-control study. Bone. 2011; 48(5):1140–1145. PMID:
21295169.

104. Bakhtiyarova S, Lesnyak O, Kyznesova N, Blankenstein MA, Lips P. Vitamin D status among patients with hip fracture and elderly control subjects in Yekaterinburg, Russia. Osteoporos Int. 2006; 17(3):441–446. PMID:
16328605.

105. Kim K, Gong HS, Lim JY, Kim JH, Baek GH. The vitamin D receptor expression in skeletal muscle of women with distal radius fracture. Arch Osteoporos. 2018; 13(1):24. PMID:
29532175.

106. Lee HJ, Gong HS, Song CH, Lee JE, Lee YH, Baek GH. Evaluation of vitamin D level and grip strength recovery in women with a distal radius fracture. J Hand Surg Am. 2013; 38(3):519–525. PMID:
23391356.

107. Visser M, Deeg DJ, Lips P. Longitudinal Aging Study Amsterdam. Low vitamin D and high parathyroid hormone levels as determinants of loss of muscle strength and muscle mass (sarcopenia): the Longitudinal Aging Study Amsterdam. J Clin Endocrinol Metab. 2003; 88(12):5766–5772. PMID:
14671166.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download