Abstract
Objectives
This study aimed to evaluate the cell viability and migration of Endosequence Bioceramic Root Canal Sealer (BC Sealer) compared to MTA Fillapex and AH Plus.
Materials and Methods
BC Sealer, MTA Fillapex, and AH Plus were placed in contact with culture medium to obtain sealers extracts in dilution 1:1, 1:2 and 1:4. 3T3 cells were plated and exposed to the extracts. Cell viability and migration were assessed by 3-(4,5-dimethyl-thiazoyl)-2,5-diphenyl-tetrazolium bromide (MTT) and Scratch assay, respectively. Data were analyzed by Kruskal-Wallis and Dunn's test (p < 0.05).
Results
The MTT assay revealed greater cytotoxicity for AH Plus and MTA Fillapex at 1:1 dilution when compared to control (p < 0.05). At 1:2 and 1:4 dilutions, all sealers were similar to control (p > 0.05) and MTA Fillapex was more cytotoxic than BC Sealer (p < 0.05). Scratch assay demonstrated the continuous closure of the wound according to time. At 30 hours, the control group presented closure of the wound (p < 0.05). At 36 hours, only BC Sealer presented the closure when compared to AH Plus and MTA Fillapex (p < 0.05). At 42 hours, AH Plus and MTA Fillapex showed a wound healing (p > 0.05).
Go to : 
References
1. Sarkar NK, Caicedo R, Ritwik P, Moiseyeva R, Kawashima I. Physicochemical basis of the biologic properties of mineral trioxide aggregate. J Endod. 2005; 31:97–100.

2. Ranjkesh B, Chevallier J, Salehi H, Cuisinier F, Isidor F, Løvschall H. Apatite precipitation on a novel fast-setting calcium silicate cement containing fluoride. Acta Biomater Odontol Scand. 2016; 2:68–78.

3. Parirokh M, Torabinejad M, Dummer PM. Mineral trioxide aggregate and other bioactive endodontic cements: an updated overview – part I: vital pulp therapy. Int Endod J. 2018; 51:177–205.

4. Silva EJ, Carvalho NK, Ronconi CT, De-Deus G, Zuolo ML, Zaia AA. Cytotoxicity profile of endodontic sealers provided by 3D cell culture experimental model. Braz Dent J. 2016; 27:652–656.

5. Rodríguez-Lozano FJ, García-Bernal D, Oñate-Sánchez RE, Ortolani-Seltenerich PS, Forner L, Moraleda JM. Evaluation of cytocompatibility of calcium silicate-based endodontic sealers and their effects on the biological responses of mesenchymal dental stem cells. Int Endod J. 2017; 50:67–76.

6. Chen I, Salhab I, Setzer FC, Kim S, Nah HD. A new calcium silicate–based bioceramic material promotes human osteo and odontogenic stem cell proliferation and survival via the extracellular signal-regulated kinase signaling pathway. J Endod. 2016; 42:480–486.

7. Yang Q, Lu D. Premix biological hydraulic cement paste composition and using the same. United States Patent Application 2008029909. 2008 Dec 4.
8. Loushine BA, Bryan TE, Looney SW, Gillen BM, Loushine RJ, Weller RN, Pashley DH, Tay FR. Setting properties and cytotoxicity evaluation of a premixed bioceramic root canal sealer. J Endod. 2011; 37:673–677.

9. Liang CC, Park AY, Guan JL. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc. 2007; 2:329–333.

10. Sjögren U, Hagglund B, Sundqvist G, Wing K. Factors affecting the long-term results of endodontic treatment. J Endod. 1990; 16:498–504.

11. Schaeffer MA, White RR, Walton RE. Determining the optimal obturation length: a meta-analysis of literature. J Endod. 2005; 31:271–274.

12. Giacomino CM, Wealleans JA, Kuhn N, Diogenes A. Comparative biocompatibility and osteogenic potential of two bioceramic sealers. J Endod. 2019; 45:51–56.

13. Victoria-Escandell A, Ibañez-Cabellos JS, de Cutanda SB, Berenguer-Pascual E, Beltrán-García J, García-López E, Pallardó FV, García-Giménez JL, Pallarés-Sabater A, Zarzosa-López I, Monterde M. Cellular responses in human dental pulp stem cells treated with three endodontic materials. Stem Cells Int. 2017; 2017:8920356.

14. Collado-González M, Tomás-Catalá CJ, Oñate-Sánchez RE, Moraleda JM, Rodríguez-Lozano FJ. Cytotoxicity of Guttaflow Bioseal, Guttaflow 2, MTA Fillapex, and AH Plus on human periodontal ligament stem cells. J Endod. 2017; 43:816–822.

15. Pinheiro LS, Iglesias JE, Boijink D, Mestieri LB, Poli Kopper PM, Figueiredo JA, Grecca FS. Cell viability and tissue reaction of NeoMTA Plus: an in vitro and in vivo study. J Endod. 2018; 44:1140–1145.

16. Almeida LH, Gomes AP, Gastmann AH, Pola NM, Moraes RR, Morgental RD, Cava SS, Felix AO, Pappen FG. Bone tissue response to an MTA-based endodontic sealer, and the effect of the addition of calcium aluminate and silver particles. Int Endod J. 2019; 52:1446–1456.

17. Khan S, Kaleem M, Fareed MA, Habib A, Iqbal K, Aslam A, Ud Din S. Chemical and morphological characteristics of mineral trioxide aggregate and Portland cements. Dent Mater J. 2016; 35:112–117.

18. Zhou HM, Shen Y, Zheng W, Li L, Zheng YF, Haapasalo M. Physical properties of 5 root canal sealers. J Endod. 2013; 39:1281–1286.

19. Camps J, About I. Cytotoxicity testing of endodontic sealers: a new method. J Endod. 2003; 29:583–586.

20. Tanomaru-Filho M, Andrade AS, Rodrigues EM, Viola KS, Faria G, Camilleri J, Guerreiro-Tanomaru JM. Biocompatibility and mineralized nodule formation of Neo MTA Plus and an experimental tricalcium silicate cement containing tantalum oxide. Int Endod J. 2017; 50(Supplement 2):e31–e39.

21. Borges RP, Sousa-Neto MD, Versiani MA, Rached-Júnior FA, De-Deus G, Miranda CE, Pécora JD. Changes in the surface of four calcium silicate-containing endodontic materials and an epoxy resin-based sealer after a solubility test. Int Endod J. 2012; 45:419–428.

22. Mahdi JG, Alkarrawi MA, Mahdi AJ, Bowen ID, Humam D. Calcium salicylate-mediated apoptosis in human HT-1080 fibrosarcoma cells. Cell Prolif. 2006; 39:249–260.

23. Silva Almeida LH, Moraes RR, Morgental RD, Pappen FG. Are premixed calcium silicate-based endodontic sealers comparable to conventional materials? A systematic review of in vitro studies. J Endod. 2017; 43:527–535.

24. ISO-Standards ISO 10993 Biological evaluation of medical devices – part 5. tests for in vitro cytotoxicity. Geneva: International Organization for Standardization;2009.
Go to : 
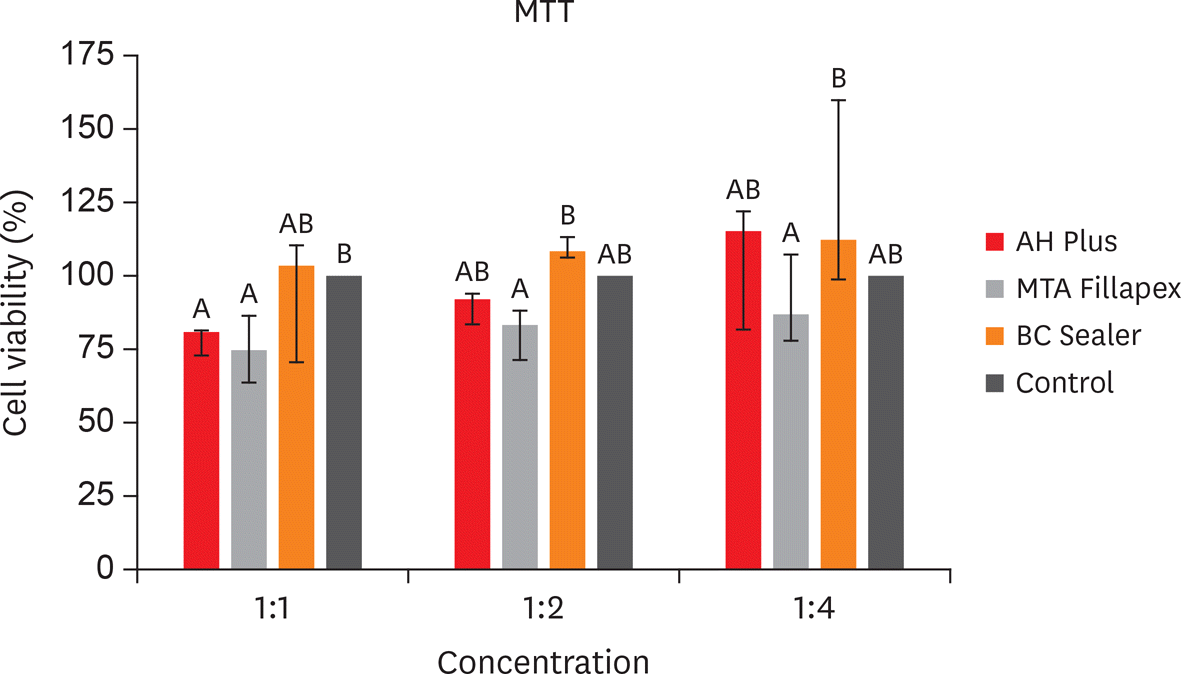 | Figure 1.Cell viability results (%) by 3-(4,5-dimethyl-thiazoyl)-2,5-diphenyl-tetrazolium bromide (MTT) assay in 3T3 cells exposed to 24 hours for AH Plus, MTA Fillapex and BC Sealer, at dilution of 1:1, 1:2 and 1:4, according to the control group (100% viability rate). Bars with different letters represent significant differences among groups in each dilution extract (p < 0.05). |
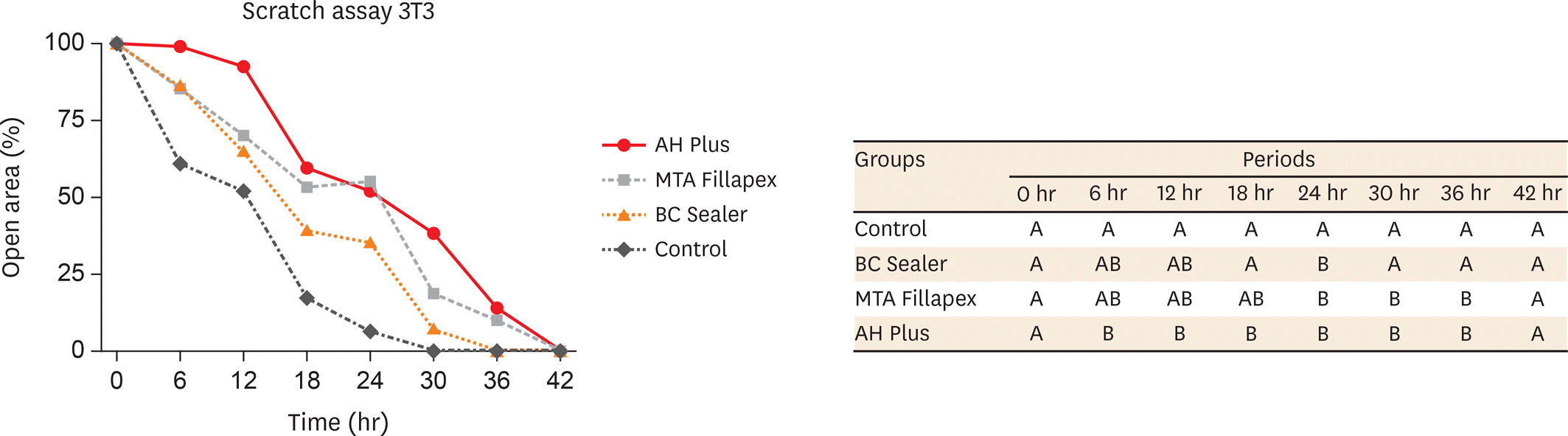 | Figure 2.Cell migration results by scratch assay in 3T3 cells exposed to set extracts of AH Plus, MTA Fillapex and BC Sealer at dilution of 1:8. Percentage of open area by scratch assay in 3T3 cells. Columns with different letters represent significant differences between groups in each evaluated period. |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download