INTRODUCTION
Prophylaxis for hepatitis B virus (HBV) recurrence is essential after liver transplantation (LT) in cases with prior association with HBV infection because this virus can replicate soon after LT. Hepatitis B immunoglobulin (HBIG) treatment has been the mainstay of HBV prophylaxis over a long period. The prophylactic mechanism of HBIG monotherapy is based on lifelong maintenance of high hepatitis B surface antibody (anti-HBs) levels to neutralized HBV. Because HBIG is expensive and viral mutations causing resistance can develop, antiviral nucleos(t)ide analog (NA) is often administered concurrently to enhance prophylactic efficacy and to reduce the financial burden through a decreased use of HBIG.
123456 This combination therapy is now accepted as one of the mainstays of post-transplant HBV prevention in many LT centers worldwide. HBIG-sparing and HBIG-free prophylaxis regimens have also recently been adopted as they are more cost-effective than conventional combination therapies.
78
In Korea, where the prevalence of HBV infection in general population is still high, a majority of adult LT recipients are associated with HBV infection. Half of adult patients in Korea undergoing living donor liver transplantation (LDLT) are diagnosed of hepatocellular carcinoma (HCC), which is often associated with preceding HBV infection. Post-transplant HCC recurrence is also closely associated with recurrence of HBV infection.
910 HBV prophylaxis is therefore one of the most important components of post-transplant patient management, with combination therapy approach usually adopted to achieve maximal prophylactic efficacy despite the high costs of these treatments.
11
We have here evaluated the current real-world practices in relation to HBV prophylaxis after LT in Korea using the patient information from the Korean Organ Transplantation Registry (KOTRY) database.
METHODS
Study scheme
This study consisted of three parts. Part 1 included analysis of the clinical parameters provided by the KOTRY LT database. Part 2 analyzed the simulative half-life (SHL) of exogenously administered HBIG using additional data from 12 participating institutions. Part 3 involved testing of the HBV viral load in peripheral blood samples taken shortly before the LT operation. These blood specimens were provided by KOTRY and the Korea Centers for Disease Control and Prevention.
Patient selection and data collection
The KOTRY LT database is a multi-center repository of LT data that have been prospectively collected since 2014. The criteria for patient selection in our present study included age ≥ 18 years, hepatitis B surface antigen (HBsAg) sero-positivity at the time of the LT operation, and post-transplant follow-up period ≥ 2 years. Exclusion criteria were concurrent hepatitis C virus infection, patient death within the first year post-transplantation, and re-transplantation. All of the included study patients were indicated for post-transplant HBV prophylaxis and followed up until July 2019. This study cohort was used for the Part 1 analysis.
Because the KOTRY LT database does not collect detailed information on HBIG administration, additional data were collected from the 12 participating institutions after obtaining IRB approval. These additional data were used in Part 2 of the present study.
Recipient blood samples are collected at the time of enrollment to the KOTRY LT database. We used these specimens to measure the HBV viral load at the time of LT operation, using real-time polymerase chain reaction (PCR) as described below. The pre-treated blood specimens were provided through the Korea BioBank Project at the Korea Centers for Disease Control and Prevention.
Institutional protocol for post-transplant HBV prophylaxis
Post-transplant HBV prophylaxis was conducted in accordance with the protocols at Asan Medical Center. Briefly, 10,000 IU HBIG was infused during the anhepatic phase, daily during the first week, weekly for the next month, and monthly thereafter. At one year after LT, the interval between regular HBIG infusions was gradually prolonged up to 6–16 weeks, on a patient-by-patient basis, to maintain the trough serum anti-HBs titers ≥ 200–500 IU/L. A high-genetic barrier NA (entecavir or tenofovir) has often been added to the combination therapy regimens.
1213 A majority of the LT centers that participated in this study used similar protocols for HBV prophylaxis. Post-transplant HBV recurrence was defined as the detection of HBV DNA in the peripheral blood after 1 month post-transplantation.
Simulative pharmacokinetic assessment of the half-life of exogenously administered HBIG
The degradation curve of exogenously administered HBIG and the formulas used to calculate its SHL are summarized in
Fig. 1, and have been described in detail elsewhere.
11 Using these formulas, the parameters required to determine the interval of HBIG administration for a certain target trough titer are body weight, gender, hematocrit, trough anti-HBs titer, and the interval between two consecutive HBIG treatments.
Fig. 1
Anti-HBs pharmacokinetics. (A) Illustration of the anti-HBs pharmacokinetics and (B) formulas used to calculate the simulative half-life of HBIG and determination of infusion intervals.
HBIG = hepatitis B immunoglobulin, anti-HBs = antibody to hepatitis B surface antigen.

Real time PCR assay to measure HBV viral load in the peripheral blood
Total HBV DNA was measured via a nested PCR protocol using primers located within the S gene of this virus. Briefly, first-round PCR was performed using the primers S1, 5′-ACTCGTGGTGGACTTCTCTC-3′ (nucleotide positions 252–271) and S2, 5′-GAACCACTGAACAAATGGCA-3′ (nucleotide positions 703–684). The second-round primers were S3, 5′-GTCTGCGGCGTTTTATCATA-3′ (nucleotide positions 381–399) and S4, 5′-GGATGGGAATACAGGTGCAA-3′ (nucleotide positions 611–592). The specificity of the primer sequences was checked using the basic local alignment search tool. The PCR reactions contained 1.25 U of ATPlite Gold (Applied Biosystems, Monza, Italy), 4 mmol/L MgCl2, 1 × gold buffer, 200 μmol/L dNTPs, and 50 pmol of each primer in a final volume of 50 μL. The PCR conditions for the first-round PCR were as follows: 94°C for 10 minutes and then 1 cycle at 94°C for 1 minute followed by 35 cycles at 50°C for 45 seconds, and 72°C for 1 minute. There was then a final extension step at 72°C for 7 minutes. The amplification conditions for the second-round were the same except that an annealing temperature of 55°C was used.
14
Because the above mentioned PCR assay did not provide absolute concentration values for the HBV viral load, blood samples from 20 of the study patients were concurrently assessed with the Abbott Realtime HBV system (Abbot Laboratories, Abbot Park, IL, USA), which has a dynamic range of between 15 and 1.1 × 108 IU/mL. Comparison of the two HBV viral load values provides a conversion factor. The relative values of the HBV viral load obtained from the real-time PCR were converted to absolute concentration values using this conversion factor.
Statistical analysis
All numerical data were reported as means ± standard deviation [SD] or as medians with ranges. Continuous variables were compared using the Student's t-test or analysis of variance. P < 0.05 was considered statistically significant. Statistical analyses were performed using SPSS version 22 (IBM, New York, NY, USA).
Ethics statement
The current study protocol was approved by the Institutional Review Board (IRB) at Asan Medical Center (IRB No. 2018-0739) and its counterpart at each of the participating institutions. Informed consent for this study was waived because informed consent was already obtained at the time of registration to KOTRY database.
RESULTS
Patient profiles and HBV prophylaxis results
The study cohort included 326 LT recipients, comprising 267 patients (81.9%) that underwent living-donor LT and 59 cases (18.1%) of deceased-donor LT. All patients had HBV infection leading to HBV-associated chronic hepatitis or liver cirrhosis. HCC was diagnosed prior to the LT procedure in 232 patients (71.2%). The mean patient age at LT operation was 53.6 ± 7.2 years (range, 23–72). The men and women patients in the study cohort numbered 255 (78.2%) and 71 (21.8%), respectively.
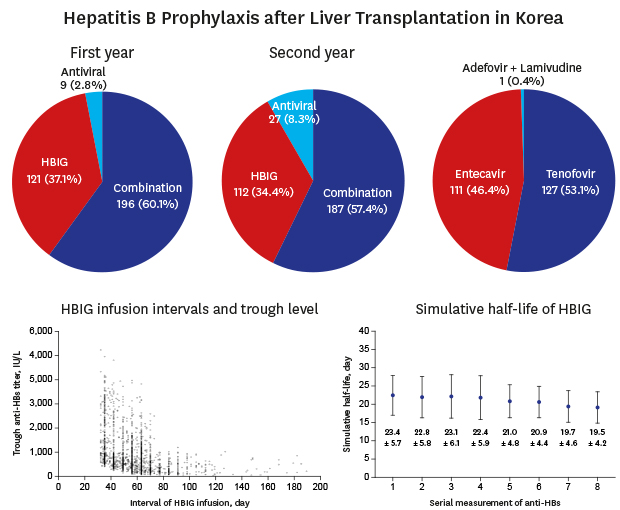
Pre-transplant NAs were administered in 255 of the study patients (78.2%) including entecavir in 126 (38.4%), tenofovir in 109 (35.5%), lamivudine in 6 (1.8%), telbivudine in 6 (1.8%), adefovir in 3 (0.9%), and adefovir plus lamivudine in 5 cases (1.5%) (
Fig. 2A).
Fig. 2
Diagrams of HBV treatment. (A) Pre-transplant treatment of HBV infection, (B) HBV prophylaxis during the first and second years post-transplant, and (C) types of antiviral agents used after transplantation.
HBV = hepatitis B virus, HBIG = hepatitis B immunoglobulin.

Post-transplant HBV prophylaxis regimens during the first year included combination therapy in 196 (60.1%), HBIG monotherapy in 121 (37.1%) and NA monotherapy in 9 patients (2.8%). Their mean age, men-to-women gender ratio and median pretransplant HBV DNA load (hospital laboratory values) were 55.4 ± 6.5 years, 153:43 and 2.2log
10 IU/mL in the combination group; 53.4 ± 6.4 years, 96:25 and 1.9log
10 IU/mL in the HBIG monotherapy group; and 44.7 ± 5.2 years, 6:3 and 2.0log
10 IU/mL in the NA monotherapy group. In the second year post-transplant, these regimens were changed to combination therapy in 187 (57.4%), HBIG monotherapy in 112 (34.4%), and NA monotherapy in 27 patients (8.3%) (
Fig. 2B).
In 196 of the patients (60.1%) receiving combination therapy during post-transplant year 1, 6 cases were converted to NA monotherapy and 3 to HBIG monotherapy in year 2. In 121 patients undergoing HBIG monotherapy in year 1, 12 cases were converted to NA monotherapy at year 2 post-transplantation. All 9 patients receiving NA monotherapy during year 1 were maintained on this protocol in year 2.
The NAs used after LT in our current study series were tenofovir in 127 (39.0%), entecavir in 111 (34.0%), and adefovir plus lamivudine in 1 patient (0.3%) (
Fig. 2C). The median post-transplant interval to NA commencement was 29 days (range, 1–1,211 days). Of the 239 patients undergoing NA medication, 211 (88.3%) received this treatment within the first 3 months after LT. The primary reason for continuing with the same pre-transplant NA after LT operation was due to the health insurance policy that free interchange between entecavir and tenofovir is not permissible unless serious adverse side-effects develop.
During the 2-year post-transplant follow-up period, HCC recurrence and HBV recurrence developed in 18 (5.5%) and 6 (1.8%) patients, respectively. HCC recurrence developed in 3 of 6 patients with HBV recurrence. Two of these patients with HBV recurrence received combination therapy just after the LT operation. The other three patients underwent HBIG monotherapy, which was converted to combination therapy after HBV recurrence. One patient underwent NA monotherapy with peri-transplant HBIG administration only. One of two patients who received combination therapy showed HBV recurrence that was associated with concurrent HCC recurrence. The detailed profiles of these six cases of HBV recurrence are summarized in
Table 1.
Table 1
Profiles of the study patients showing post-transplant HBV recurrence

|
Age at LT, yr |
Gender |
Concurrent HCC at LT |
Pretransplant HBV load, IU/mL |
Pretransplant antiviral agent |
Date of LT |
Type of LT |
Initial HBV prophylaxis |
HBV prophylaxis after second year |
Date of HBV recurrence |
Interval between LT and HBV recurrence, day |
HCC recurrence |
Date of HCC recurrence |
Posttransplant antiviral agent |
Start date of posttransplant antiviral agent |
Interval between HBV recurrence and antiviral agent, day |
Patient survival |
|
58 |
Men |
Absent |
116 |
None |
2014-12-14 |
DDLT |
Antiviral agent |
Antiviral agent |
2014-12-14 |
30a
|
No |
|
Tenofovir |
2015-01-27 |
44 |
Alive |
|
54 |
Men |
Present |
116 |
None |
2014-12-03 |
LDLT |
HBIG → Combination |
Antiviral agent |
2016-04-14 |
498 |
Yes |
2016-09-22 |
Entecavir |
2015-01-09 |
−461 |
Alive |
|
50 |
Men |
Absent |
116 |
None |
2015-04-09 |
DDLT |
Combination |
Antiviral agent |
2016-05-03 |
390 |
No |
|
Entecavir |
2015-05-01 |
−368 |
Alive |
|
46 |
Men |
Present |
15 |
Entecavir |
2015-10-22 |
LDLT |
HBIG |
Combination |
2016-06-03 |
225 |
Yes |
2017-11-25 |
Tenofovir |
2016-07-19 |
46 |
Alive |
|
53 |
Men |
Present |
100 |
Tenofovir |
2015-08-03 |
LDLT |
Combination |
Combination |
2016-09-27 |
421 |
Yes |
2016-11-17 |
Tenofovir |
2015-08-18 |
−406 |
Alive |
|
52 |
Men |
Present |
15 |
Entecavir |
2015-01-22 |
LDLT |
HBIG |
HBIG |
2017-09-17 |
969 |
No |
|
Tenofovir |
2017-09-22 |
30 |
Alive |
Analysis of the pre-transplant HBV viral load
Prior to the LT operation, the HBV viral load assessed by each institutional laboratory was undetectable in 69 patients (21.2%), and was 15–100 IU/mL in 111 patients (34.1%), 101–1,000 IU/mL in 75 patients (23.0%), 1,001–10,000 IU/mL in 25 patients (7.7%), and >10,000 IU/mL in 45 patients (13.8%). Among the entire cohort of 326 patients, blood samples had been taken shortly before the LT procedure in 325 patients and were assayed using real-time PCR. HBV DNA was detected by PCR in all 325 blood samples as follows: detectable but <15 IU/mL in 148 patients (45.5%), 15–100 IU/mL in 18 patients (5.5%), 101–1,000 IU/mL in 68 patients (20.9%), 1,001–10,000 IU/mL in 56 patients (17.2%), and >10,000 IU/mL in 35 patients (10.8%). Comparisons of these measurements stratified by institutional laboratory and our real-time PCR assay values are depicted in
Fig. 3. No significant correlation was found between the HBV viral loads determined by the institutional laboratories and by our current PCR assays (institutional laboratory value = 1.7382 – 0.0003 × PCR value;
r = 0.0003, r
2 = 0.0000,
P = 0.996) (
Fig. 4).
Fig. 3
Diagrams of HBV DNA concentration. (A) Distribution of pre-transplant HBV DNA concentrations in the peripheral blood, assessed by the institutional laboratories and (B) real-time PCR analysis in this study.
“Undetectable” in the right graph denotes a HBV DNA concentration of less than 84 copies/mL or 15 IU/mL.
RT-PCR = reverse transcription polymerase chain reaction, HBV = hepatitis B virus, PCR = polymerase chain reaction.
Fig. 4
Scatter plot showing the association between the hospital laboratory HBV DNA concentration and real-time PCR detection used in this study.
HBV = hepatitis B virus, PCR = polymerase chain reaction.

Assessment of HBIG SHL
The SHL of exogenously administered HBIG in the 280 patients who received HBIG monotherapy or combination therapy was assessed. We excluded 46 cases due to short-term use of HBIG only or incompletely collected data. The association between HBIG infusion interval and anti-HBs trough titer in 2,327 anti-HBs trough titer measurements is depicted in
Fig. 5. These anti-HBs trough titers were stratified as ≤ 100 IU/mL in 15 sessions (0.7%), 101–200 IU/mL in 124 sessions (5.3%), 201–500 IU/mL in 753 sessions (32.4%), 501–1,000 IU/mL in 849 sessions (36.5%), 1,001–2,000 IU/mL in 361 sessions (15.5%), and > 2,000 IU/mL in 225 sessions (9.7%). The session numbers of anti-HBs trough titers > 500 IU/mL and > 1,000 IU/mL were 1435 (61.7%) and 586 (25.2%), respectively.
Fig. 5
Scatter plot showing the association between the HBIG infusion intervals and anti-HBs trough titers.
HBV = hepatitis B virus, HBIG = hepatitis B immunoglobulin, anti-HBs = antibody to hepatitis B surface antigen.

The individual SHL ranged from 11.6 ± 1.4 to 45.5 ± 9.2 days, with a mean of 21.1 ± 3.9 days and a median of 17.9 days. The mean coefficient of variation (CV) of the SHL was 17.3% for intra-individual variability and 18.6% for inter-individual variability
To evaluate a pool of data consistently, we analyzed a sub-cohort of 152 patients for whom 8 or more consecutive measurements of the trough anti-HBs level were available. The serial changes in these measurements are depicted in
Fig. 6A. The individual SHL ranged from 11.6 ± 1.4 to 45.5 ± 9.2 days, with a mean of 21.6 ± 4.3 days and a median of 17.7 days. The mean CV of SHL was 17.9% for intra-individual variability and 19.8% for inter-individual variability. The fluctuation in 8 consecutive sessions of trough anti-HBs level is depicted in
Fig. 6B.
Fig. 6
Sequential changes in the simulative half-life of exogenously administered hepatitis B immunoglobulin in accordance with the serial anti-HBs titer measurements. (A) Each line indicates changes in an individual patient. (B) Mean values with standard deviation are depicted.

Correlation between the HBIG SHL and HBV DNA viral load
The influence of the real-time PCR-measured pre-transplant HBV DNA load on SHL was assessed in 148 of the abovementioned 152 patients. The mean SHL was 18.9 ± 4.3 days in 56 patients showing undetectable HBV DNA (< 15 IU/mL or 84 copies/mL); 20.8 ± 5.3 day in 19 patients showing HBV DNA ≤4log10 IU/mL; 23.3 ± 5.8 days in 55 patients showing HBV DNA > 4log10 and ≤ 6log10 IU/mL; and 22.5 ± 5.6 days in 18 patients showing HBV DNA > 6log10 IU/mL (P = 0.071). In two-group comparison with 19 patients showing HBV DNA ≤ 4log10 IU/mL and 73 patients showing HBV DNA > 4log10, their mean SHLs were 20.8 ± 5.3 days and 24.0 ± 5.8 days, respectively (P = 0.19).
Correlation between HBIG SHL and post-transplant NA administration
The influence of the post-transplant NA treatment load on the HBIG SHL was assessed in 141 patients out of the abovementioned 152 patients. Patients with post-transplant HBV recurrence were excluded. The mean SHL was 19.9 ± 6.8 days in 87 patients receiving combination therapy and 23.8 ± 7.9 days in 64 patients receiving HBIG monotherapy (P < 0.001).
DISCUSSION
Our current study was primarily undertaken because we recognized that the post-transplant HBV prophylaxis protocols in Korea have moved toward combination therapy, but the HBIG infusion regimens have remained unchanged in many Korean LT centers.
11 HBV prophylaxis regimens are initially commenced with HBIG monotherapy. After introduction of potent NAs, HBV prophylaxis regimens became more diverse and included combination therapy, HBIG monotherapy, HBIG-sparing or HBIG-free therapy, and NA monotherapy. A combination therapy with high-dose HBIG and high genetic-barrier NA is regarded by clinicians as the most potent prophylaxis regimen, but its high cost remains a barrier to wider use. To decrease the financial burden of expensive HBIG treatment, various alternative regimens have been developed that reduce the usage of HBIG without sacrificing the efficacy of HBV prophylaxis.
A combination therapy for HBV prophylaxis was adopted in 60.1% of our current study patients, thus being the most preferred approach. When we excluded the patients from one institution (Asan Medical Center) where HBIG monotherapy has been the preferred treatment for a long period.
911 the proportion of patients receiving combination therapy increased to 67.5%. There are two possible reasons for this. The first is that HBIG administration requires a small financial burden on Korean patients (60–120 US dollars per 10,000 IU HBIG) because it is cheaper in Korea than other countries and 90%–95% of the medical cost for HBIG administration is covered by social health insurance. The second possible reason was the high prevalence (71.2%) of HCC in HBV-associated LT recipients among our study patients. HCC recurrence is a well-known risk factor of HBV recurrence,
9 and a potent prophylaxis regimen including high genetic barrier NA is therefore highly recommended in these cases. Consistently, NA was used after LT in 74.6% and 69.1% of the patients with and without HCC respectively in our present study population. Considering the recent trend toward combination therapy in many Korean LT centers, it would be expected that the number of LT recipients receiving combination therapy will increase gradually. From the standpoint of LT recipients in Korea, there is also no reason to not administer combination therapy because the financial burden is no longer a critically limiting factor as stated above.
However, it must be noted that the health insurance framework in Korea has the potential to cause an overdosing of HBIG.
11 As shown in
Fig. 5, 61.7% and 25.2% of the measurement sessions showed a trough anti-HBs titer above 500 IU/mL and 1,000 IU/mL, respectively. Considering that many institutions set a target for this titer of 200–500 IU/mL, it is likely that many Korean patients are administered HBIG too much or too frequently. These trough anti-HBs titers do fluctuate from time to time however, making it difficult to appropriately adjust the HBIG infusion intervals in the clinic. To optimize these infusion intervals, we have developed the concept of an HBIG SHL which does not require complex pharmacokinetic blood sampling to measure and uses readily obtained clinical parameters such as gender, body weight and the hematocrit level. In our current sub-cohort of 152 patients, the mean SHL was 21.6 ± 4.3 days with a median of 17.7 days. The mean CV of the SHL of 17.9% for intra-individual variability and 19.8% for inter-individual variability. These values were comparable to our previous single-institution results showing a mean SHL of 19.9 ± 2.8 days with a mean CV of 14.0% ± 3.4% and 19.0% ± 1.3% for intra- and inter-individual variability, respectively.
11
We investigated the factors affecting SHL but found no association between the mean SHL and pre-transplant HBV DNA load. In contrast, the SHL was significantly longer in patients undergoing HBIG monotherapy comparing with those receiving combination therapy. This difference appears to be due to a selection bias. Our previous study found no differences in the SHL according to the HBV prophylaxis regimens used.
11 Considering that the pre-transplant HBV DNA load is greatly influenced by pre-transplant NA use and nearly no consumption of HBIG by HBV per se without HBV recurrence, the degradation speed of exogenously administered HBIG appears to be a kind of constitutional factor.
The concept of individualized HBIG SHL makes it possible to adjust the HBIG infusion interval appropriately. By applying the SHL and related formulas to a certain target anti-HBs trough titer, the HBIG infusion interval can be shortened or lengthened in 2 week steps. In our earlier prospective study with a target titer of 200 IU/mL, the HBIG infusion interval was successfully increased to 12 weeks or longer in 99 of 114 patients (86.8%).
11 We believe that more than 50% of our current study patients would have benefited from an increased HBIG infusion interval, appropriately adjusted using the HBIG SHL. This would of course have the benefit of requiring fewer outpatient visits.
A small number of our study patients appeared to receive NA monotherapy intentionally as a part of clinical study. There have been some prior studies on HBIG-free and HBIG-sparing HBV prophylaxis regimens.
78 Theoretically, HBIG-free NA prophylaxis cannot prevent intraoperative HBV infection of naïve graft hepatocytes unless a sustained virological response (SVR) is achieved before LT operation. Hence, it is reasonable to perform HBIG-free NA prophylaxis only in patients who show SVR prior to LT. In a previous study on entecavir monotherapy from the University of Hong Kong,
7 HBsAg sero-clearance was reported to occur within 1 year in 90% of cases, but reappear in 13% of these patients at 3 years. At 1 year post-LT in the study population, the HBsAg sero-clearance rates were 98%, 92%, 81%, and 60% for HBV DNA levels of undetectable, ≤ 4log
10, > 4–6log
10, and > 6log
10 IU/mL, respectively (
P < 0.001). The authors of this study have commented that additional HBIG administration would invariably lead to a decreased HBsAg, thus supporting the benefit of HBIG-sparing prophylaxis rather than HBIG-free regimens.
8
Given that HBIG-sparing and HBIG-free regimens were developed to reduce the cost burdens of HBIG administration, they do not appear to be widely recommended in the current Korean transplantation setting. It is notable in this regard that one patient receiving NA monotherapy in our current series showing HBV recurrence without HCC recurrence would not have experienced this outcome if combination therapy had been performed. A prior meta-analysis of 19 studies, incorporating 1,484 patients, has indicated that HBIG administration reduces the rate of HBV recurrence and viral mutants, with further subgroup analysis indicating that HBIG was necessary to reduce the HBV recurrence rate in patients with positive pre-transplant HBV DNA status.
15
It was of interest that 21.2% of our current study patients were reported to have an undetectable HBV DNA titer, but we successfully identified HBV DNA in the blood samples of all patients using real-time PCR assays, although 45.5% showed a low HBV DNA titer that was below the usual the detection limit (15 IU/mL or 84 copies/mL). This finding is compatible with the general concept that HBV is present in patients showing NA-induced SVR. This indicates that HBV neutralization by HBIG is required for a prolonged period.
16
This study had some limitations of note. First, although this was a prospective cohort study, the sourcing of the data made this study a retrospective multicenter investigation. Second, the details of the institutional protocols for HBV prophylaxis were not analyzed. Third, the follow-up period was short and the incidence of HBV recurrence was low and risk factor analysis for HBV recurrence was therefore not carried out.
In conclusion, combination therapy regimen is the mainstay of post-LT HBV prophylaxis in Korea due to the available medical insurance coverage and high prevalence of HCC. Excessive HBIG has often been administered which is wasteful. Individualized optimizations of HBIG administration using SHL are therefore desirable to appropriately adjust the infusion intervals.








 PDF
PDF Citation
Citation Print
Print






 XML Download
XML Download