Abstract
Obesity results in an inflammatory microenvironment in adipose tissue, leading to the deterioration of tissue protective mechanisms. Although recent studies suggested the importance of type 2 immunity in an anti-inflammatory microenvironment in adipose tissue, the regulatory effects of T helper 2 (Th2) cytokines on systemic metabolic regulation are not fully understood. Recently, we identified the roles of the Th2 cytokine (interleukin 4 [IL-4] and IL-13)-induced adipokine, growth differentiation factor 15 (GDF15), in adipose tissue in regulating systemic glucose metabolism via signal transducer and activator of transcription 6 (STAT6) activation. Moreover, we showed that mitochondrial oxidative phosphorylation is required to maintain these macrophage-regulating autocrine and paracrine signaling pathways via Th2 cytokine-induced secretion of GDF15. In this review, we discuss how the type 2 immune response and Th2 cytokines regulate metabolism in adipose tissue. Specifically, we review the systemic regulatory roles of Th2 cytokines in metabolic disease and the role of mitochondria in maintenance of type 2 responses in adipose tissue homeostasis.
The mammalian immune response can be roughly divided into two categories. The type 1 immune response is an acute, highly inflammatory program intended to activate phagocytic cells, such as neutrophils, T helper type 1 (Th1) cells, and type 1 macrophages (M1) to destroy the targets, and is often associated with tissue damage [1]. The type 1 immune response is strongly facilitated by interleukin 2 (IL-2), interferon γ (IFN-γ), and lymphotoxin-α secreted by Th1 lymphocytes. In contrast, the type 2 immune response is a more long-lived program that plays a major role in barrier defenses, such as intestinal motility and mucus barriers, and tissue remodeling, including fibrosis and wound healing [2]. The type 2 immune response is characterized by T helper 2 (Th2) cells, eosinophils, mast cells, basophils, type 2 innate lymphoid cells (ILC2s), IL-4 and/or IL-13-induced macrophages, immunoglobulin E (IgE), and the cytokines IL-4, IL-5, IL-9, and IL-13 [23]. When the Th1–Th2 dichotomy was first described [4], type 2 immunity was generally considered a regulatory function, not as an important response to pathological conditions, and its primary function was to limit the injurious consequences of type 1-mediated immunity [5]. Although this original limited definition remains accurate, recent studies have found that the role of type 2 immunity includes acting as a major effector response that has many important host-protective and pathogenic activities, in addition to suppressing type 1 immunity and type 1-driven inflammation [6]. In addition, these cell populations also show protective activity by reducing inflammation in the process of tissue regeneration [7].
Many recent studies have focused on the immune regulation of adipose tissue (AT) to achieve systemic glucose homeostasis in metabolic disease. AT can be broadly divided into two categories: brown adipose tissue (BAT), which is a highly catabolic tissue that undergoes high levels of thermogenesis, and white adipose tissue (WAT), an anabolic tissue that serves as a primary long-term nutrient storage site [89]. AT is comprised of stromal and vascular cells, including innate and adaptive immune cells, fibroblasts, and endothelial cells in addition to adipocytes, and recent research has focused on immune mediators as a major axis of metabolic regulation [101112]. Previously, AT in the obese state was shown to promote inflammation mediated by type-1 signals, including infiltration and expansion of M1 macrophages with secretion of inflammatory cytokines, such as tumor necrosis factor-α (TNF-α), IL-6, and IL-1β. However, more recent studies have identified the pivotal role of type 2 immunity, including regulatory T (Treg) cells, Th2 cells, and ILC2 cells, which suppress inflammatory responses by production of the anti-inflammatory cytokine, IL-10, and contribute to the development of insulin resistance [13141516]. There is emerging evidence that the IL-33–driven ILC2/eosinophil axis plays a role in browning of WAT, and eosinophils in AT are involved in metabolic homeostasis via IL-4/IL-13–mediated reconstitution of macrophages, thereby preventing development of obesity [1718]. Although there has been pioneering work on the regulatory role of the ILC2 population in AT, such as the recovery of ILC2 in obesity by injection of IL-33 in obese mice [19], the mechanisms underlying the loss of ILC2 by metabolic stress have not been identified [20]. Although ILC2-derived cytokines have been shown to induce browning of WAT, how ILC2-derived cytokines affect systemic glucose metabolism is not well understood [2122].
In this review, we discuss how the type 2 immune response and Th2 cytokines regulate metabolism in AT, its most well-studied context. Specifically, we review the systemic regulatory roles of Th2 cytokines in metabolic disease and the role of mitochondria in maintenance of type 2 responses in AT homeostasis. We also briefly discuss the pathological consequences of their dysfunction.
Recent observations have shown that obesity results in an inflammatory microenvironment in AT, leading to the deterioration of tissue-protective mechanisms that reduce the negative outcomes of immunopathology. Although AT in lean mice and humans contains a higher proportion of immune cells associated with type 2 immunity, type 2 immune cells are reduced in number during the development of obesity [2324]. Recent studies have suggested the importance of ILC2s as central regulators of type 2 immunity in an anti-inflammatory microenvironment in AT [2526]. However, the regulatory effects of ILC2-derived Th2 cytokines with systemic metabolic regulation are not fully understood. In this section, we review the role of type 2 immune cells, including anti-inflammatory macrophages, ILC2, and eosinophils, in physiological AT homeostasis.
Adipose tissue macrophages (ATMs) are abundant immune cells in human and murine AT [2728]. ATMs, which have substantial functional heterogeneity, actively participate in various aspects of nutrient metabolism within AT [29]. Macrophages identified in lean AT are anti-inflammatory “M2”, or alternatively activated (M2-like) macrophages, which typically express the mannose receptor CD206 and secrete anti-inflammatory cytokines (Fig. 1) [3031]. AT in lean mice and humans contains a higher proportion of M2/M1 macrophages, which are associated with local production of Th2 cytokines by eosinophils [1832]. Initially, the involvement of the immune response in AT homeostasis was understood only in the context of type 1 immunity, represented by the increased proinflammatory M1 macrophages and production of IFN-γ and chemokines, such as CCL2 released from CD8+ and Th1-polarized lymphocytes, as well as their ability to exacerbate metabolic dysfunctions, such as type 2 diabetes mellitus and obesity [33].
M2 macrophages play a role in nutrient storage in adipocytes by promoting insulin sensitivity through the secretion of IL-10 [34], and suppress type 1 immune responses of leukocyte populations by processing excess iron, disposing of dead cells and debris, and promoting vascularization and tissue matrix remodeling [3536]. The regulatory key mediators of macrophage polarization were recently identified [37]. M1 macrophages have huge metabolic demands depending on glycolysis [3839]. M2 macrophages meet their energy requirements using mitochondrial oxidative phosphorylation (OXPHOS) pathways, and have increased ability to uptake free fatty acid and for fatty acid oxidation (FAO) to facilitate the tricarboxylic acid cycle (TCA) cycle, and drive inflammasome activation in inflammatory macrophages [3740]. Inhibition of FAO in M2 macrophages with the carnitine palmitoyltransferase 1 (CPT1) inhibitor, etomoxir, limits M2 activation in response to IL-4 [34]. Peroxisome proliferator-activated receptor γ/δ (PPAR-γ/δ), which can be driven by adipocyte derived IL-4 and IL-13, is a crucial transcription factor in M2 macrophage polarization [4142]. In contrast, M1 macrophages increase energy production through glycolysis. M1 macrophages also have a “broken” TCA cycle, resulting in accumulation of citrate and succinate. Increased succinate results in secretion of the proinflammatory cytokine, IL-1β, via activation of hypoxia-inducible factor 1α (HIF1α) (Fig. 1) [404344]. Blocking oxidative metabolism not only impairs the development of an M2-like phenotype but also drives the cells toward an M1-like state [45]. In addition, failure of alternative M2 activation, which is associated with reduced oxidative function, leads to classical macrophage activation, elevated weight gain, and obesity with concurrent adipose inflammation and insulin resistance [4647]. However, it is unclear whether the oxidative function of macrophages is reduced under these conditions, and it is not known whether treatments capable of increasing the oxidative function of macrophages would reverse insulin resistance and adipose inflammation.
ILC2s are a recently identified innate immune cell lineage related to lymphocytes and natural killer cells with emerging roles in mediating immune responses and regulating tissue homeostasis and inflammation [48]. The group 2 ILC population is comprised of ILC2s, which express IL-5 and IL-13 and require GATA-binding protein 3 (GATA3) and retinoic acid receptor-related orphan receptor α (ROR-α) for their development [49]. In a mouse model of intestinal damage and inflammation, the epithelial cytokine IL-33 can promote ILC2 amphiregulin (Areg) production, leading to the resolution of colitis and promoting epithelial repair [50]. Indeed, type 2 immune responses are known to promote wound repair and tissue regeneration following infection or injury [1], suggesting that ILC2s may be key organizers of these beneficial tissue responses. Although they have crucial roles in protective type 2 immunity, the role of these cells in WAT is quite distinct [51]. They function to promote WAT homeostasis through several defined mechanisms.
ILC2s are present in visceral adipose tissue (VAT), where they are the predominant producers of IL-5 and IL-13 following prolonged exposure to IL-33 [2552]. In lean AT, IL-33 drives the recruitment and/or proliferation of ILC2, but the cellular origin of IL-33 and the mechanisms leading to its secretion in homeostasis remain poorly understood. Tissue ILC2s are key producers of systemic IL-5 required for homeostatic eosinophil maintenance [53]. In AT, secretion of IL-5 by ILC2 is essential for the recruitment and maintenance of eosinophils and is dependent on IL-33 [53]. In addition, the most well-studied mechanism involves their regulatory effect on the resident macrophage phenotype from M1 macrophages to M2 macrophages by IL-4 or IL-13 signaling [54]. The regulatory function of these Th2 cytokines is involved in systemic glucose homeostasis. IL-4 and IL-13, both induced by type 2 immune responses, reduce inflammation in AT and improve systemic glucose intolerance by inducing polarization of M2 macrophages [185556]. Interestingly IL-33 has been shown to be competent to induce macrophage proliferation independently of IL-4Ra expression in other non-adipose macrophage populations [53], but whether IL-33 can directly activate ATMs remains to be investigated.
Eosinophils are a type of granulocyte that combat parasitic infection and allergic reactions. However, in AT their role is to maintain metabolic homeostasis [18]. Initial studies in WAT showed that eosinophils are associated with lean healthy WAT and promote M2-like polarization of WAT macrophages [1825]. WAT eosinophils decline with weight gain in association with increased AT M1-like macrophages and metabolic impairments, and eosinophil-deficient mice are more vulnerable to the onset of insulin resistance [18]. Further research has shown that WAT eosinophil numbers are maintained by IL-5, which is largely secreted by local ILC2s [1825]. One study showed that body weight and glucose control are improved by ILC2 and natural killer T cells in a model of high fat diet-induced obesity, which was attributed to the accumulation of eosinophils and M2-like macrophages in visceral WAT [57]. Recently, it was found that resident eosinophils expressing IL-4 and ILC2s expressing IL-13 are necessary to maintain an anti-inflammatory state in WAT [1825]. During cold exposure, eosinophils may induce WAT beiging by polarizing M2-like macrophages [5859]. These results suggest that ILC2s, eosinophils, and M2-like macrophages influence metabolic health through the regulation of AT inflammation, beiging capacity, and insulin resistance by facilitating a network of immune cell interactions (Fig. 1). In the simplest terms, AT M2-like macrophages, eosinophils, and ILC2s are positively correlated with AT health.
Treg cells are abundant in the visceral AT of lean mice, where they have a distinct transcriptome and antigen-receptor repertoire [60]. Similar to ILC2s, Treg cells rapidly populate AT after birth. AT Treg cells express high levels of CD25 and IL-10, as well as the transcription factors GATA3 and PPAR-γ, and the IL-33 receptor [15]. They uniquely depend on these molecules, since mice lacking IL-33, IL-33R, or PPAR-γ expression in Treg cells are deficient in Treg cells specifically within AT and exhibit evidence of increased AT inflammation [3252]. In addition, IL-33-driven expansion of ILC2s and/or Treg cells has been shown to revert the chronic inflammatory processes that drive obesity and improve insulin resistance in mouse models [61].
Recent findings support a regulatory role for type 2 immunity in AT and systemic metabolism, although the precise mechanisms remain to be determined. The possible mechanisms for the effects of type 2 immunity on AT metabolism include local effects, such as AT browning, AT remodeling, and limiting excessive type 1 inflammatory response, and systemic effects through the production of adipocytokines. In this section, we discuss the local effects of type 2 immunity in AT inflammation.
Obesity is associated with local adipose inflammation, which is characterized by dysregulated immune cell function [62]. CD8+ T cells play a critical role in high-fat diet-induced obesity and adipose inflammation via macrophage recruitment and activation [63]. ATMs secrete inflammatory cytokines, such as TNF-α and IL-6. Secretion of TNF-α, plasminogen activator inhibitor-1, IL-6, IL-1β, and other inflammatory cytokines by ATs is higher in obese patients than in lean individuals [6465]. As discussed below in the section on AT homeostasis, emerging evidence suggests that obesity is related to weakening of the type 2 immune response, since transitioning of the macrophage phenotype is likely a key mechanism by which IL-4 and/or IL-13 protect against metabolic syndrome. Accordingly, other pathways that promote M2 macrophages are also associated with metabolic homeostasis, such as the fatty acid-sensing PPAR-γ/PPAR-δ, adenosine receptor 2B, and Krüppel-like factor 4 (KLF4) pathways [666768]. Macrophage-specific deletion of PPAR-γ or PPAR-δ increases obesity, decreases glucose tolerance, and increases insulin resistance in mice fed high-fat diets [46]. Consequently, oxidative functions of ATMs control the polarization of M1-like and M2-like phenotypes, but whether reduced macrophage oxidative function causes systemic insulin resistance in vivo is not fully understood.
Recent studies have suggested a role for IL-4/IL-13–mediated reconstitution of macrophages by eosinophils in AT [18]; furthermore, administration of recombinant IL-4 to mice also reduces weight gain and has a protective effect against diet-induced obesity [55]. Importantly, IL-4 improves the metabolic indices of insulin resistance and improves the action of insulin in the liver and muscle in a signal transducer and activator of transcription 6 (STAT6)-dependent manner [55]. Although the increase in WAT eosinophils by recombinant IL-5 treatment is not related to lipid and glucose metabolism, IL-33/IL-25 upregulates WAT ILC2, which recruits eosinophils via IL-5, resulting in increased M2 macrophages and improved WAT homeostasis [57], suggesting a pivotal role for ILC2 in glucose homeostasis [69]. In addition, cold or exogenous IL-33 stimulates ILC2 and eosinophil production of IL-13 and IL-4, respectively, which drive the proliferation and commitment of adipocyte precursors to beige fat [5970]. IL-33-activated ILC2s produce methionine-enkephalin peptides, which act directly on adipocytes to stimulate beige adipogenesis independently of eosinophils [17]. These results suggest that type 2 immunity has a regulatory role in beige adipogenesis via Th2 cytokines, influencing the generation and maturation of adipocyte precursors for beige adipocyte differentiation, and supporting thermogenic activation to improve energy metabolism.
Emerging evidence supports a role of Th2 cytokines as a surrogate factor to stimulate the proliferation of M2 macrophages associated with low-grade inflammation in AT and differentiation of adipose precursors in metabolic homeostasis. A number of studies have established other effects of Th2 cytokines, including IL-33, IL-4, and IL-13, on adipokine secretion by adipocytes.
Accordingly, adipokines are considered to be regulators of whole-body homeostasis [71]. In addition to leptin, TNF-α, IL-6, resistin, FGF21, and adiponectin, which have been extensively reviewed, and a number of other key adipokines are involved in the regulation of inflammation [72737475]. We showed that growth differentiation factor 15 (GDF15) is a useful predictive biomarker of cardiovascular risk in newly diagnosed T2D patients [767778]. In addition, we identified the significance of neuregulin 4 (Nrg4) as a biomarker that is positively correlated with serum glucose level and insulin resistance in type 2 diabetes mellitus patients [77]. Kang et al. [78] reported significant increases in proinflammatory cytokine levels, such as monocyte chemoattractant protein-1 and TNF-α, in the VAT of patients with modest obesity and early metabolic dysfunction. Serum levels of adiponectin and leptin were significantly associated with insulin resistance and obesity. However, there were no obvious changes in macrophage phenotype or macrophage infiltration in patients with modest obesity or early metabolic dysfunction [78]. We showed that the expression of CD163/CD68, a marker of macrophage infiltration, was significantly related to mitochondrial biogenesis-associated genes, such as proliferator-activated receptor-γ coactivator 1α (PGC-1α), PGC-1β, and OXPHOS. Previous studies have also suggested that alternative activation of FAO and mitochondrial biogenesis in macrophages by PGC-1β may be related to obesity [7980]. Consequently, these findings suggest that the regulation of M2 macrophages in obesity may be closely linked to mitochondrial biogenesis, and that changes in the production of inflammatory biomolecules precede increased immune cell infiltration and induction of a macrophage phenotype switch in modest obesity in humans.
To explore the effects of Th2 cytokines on adipokines in AT homeostasis, we examined Th2 cytokine-mediated release of adipokines [81]. In vivo administration of α-galactosylceramide or IL-33 increased IL-4 and IL-13 production, thereby increasing GDF15 levels in AT and plasma in a STAT6-dependent manner using a STAT6 knockout mouse model. In addition, we found that administration of recombinant IL-13 to wild-type mice fed a high-fat diet improved glucose intolerance. Using GDF15-knockout mice, we showed that this effect was dependent on GDF15 expression. We found that Th2 cytokines regulate GDF15, and our results suggested that GDF15 is required for Th2 cytokine-induced improvement of glucose intolerance in metabolic disease [81].
GDF15, also known as macrophage-inhibiting cytokine 1, belongs to the transforming growth factor-β superfamily and is highly expressed in the heart, liver, kidneys, and colon [39]. Recently, the metabolic effects of GDF15 were found to include regulation of appetite by the orphan receptor, GFRAL [8283]. Moreover, recent evidence supports an important role of GDF15 in peripheral organs, including the liver, WAT, and muscle; GDF15 regulates systemic glucose tolerance as a mitochondrial unfolded protein response-associated critical cell nonautonomous myomitokine by promoting oxidation and lipolysis [84858687]. Further research is needed to identify peripheral receptors of GDF15 to elucidate the previse role of GDF15 in metabolic homeostasis.
Increased glycolysis has been observed in lipopolysaccharide-stimulated macrophages [83]. A series of recent studies demonstrated that commitment to Warburg metabolism equips macrophages to fulfill their effector functions, such as production of reactive oxygen species (ROS) or nitric oxide, phagocytosis, and secretion of inflammatory mediators in the context of bacterial infection [40]. Upon activation with proinflammatory stimuli, such as lipopolysaccharide or IFN-γ, macrophages undergo metabolic reprogramming and exhibit increased rates of glycolysis and decreased OXPHOS. By diverting adenosine triphosphate generation from OXPHOS to glycolysis, mitochondria become available for ROS production [88].
The type 2 cytokines, IL-4 and IL-13, promote maturation into alternatively activated macrophages, and this effect is dependent on STAT6 activation [5]. Upon IL-4 stimulation, macrophages increase fatty acid uptake, β-oxidation, and OXPHOS; these metabolic rearrangements are initiated by STAT6 and peroxisome PGC-1β [88]. Moreover, STAT6 and its associated transcription factors, including PPAR-γ, PPAR-δ, and PGC-1α, critically influence M2-like macrophages by increasing oxidative metabolism and mitochondrial biogenesis [6789]. Studies in mice with macrophage-specific deletion of PPAR-γ have shown that PPAR-γ is required for maturation of alternatively activated macrophages [89]. Inhibition of FAO in M2 macrophages with the CPT1 inhibitor, etomoxir, limits M2 activation in response to IL-4 [90]. Blocking oxidative metabolism not only impairs the development of an M2-like phenotype, but also drives the cells toward an M1-like state [45].
A recent study showed that blockade of lysosomal lipolysis during Heligmosomoides polygyrus infection results in defective clearance of the pathogen and inhibits commitment to OXPHOS by macrophages [90]. While changes in β-oxidation are the most striking metabolic adaptations in response to IL-4, metabolomic studies have revealed that glycolysis and glutaminolysis also contribute to TCA activity [91]. Recent research has focused on whether the oxidative function of macrophages is reduced under these conditions, and if so, whether such impairment induces insulin resistance. Using a mouse model, we found that defective oxidative function in macrophages due to myeloid-specific loss-of-function of the CR6-interacting factor 1 (Crif1) gene, an essential mitoribosomal factor required for biogenesis of OXPHOS subunits, results in systemic insulin resistance associated with adipose inflammation [92]. Moreover, macrophages from these mice are deficient in the release of GDF15, which is required for oxidative metabolism in M2-like macrophages stimulated with IL-4 and the PPAR-γ agonist, rosiglitazone. In addition, GDF15-deficient macrophages underwent polarization into an M1-like phenotype, and reintroduction of GDF15-null macrophages into high-fat diet-fed mice in which macrophages were depleted with clodronate resulted in glucose intolerance. We found that the mitochondrial oxidative function of macrophages has an important role in GDF15 secretion, and dysfunction of mitochondria in macrophages causes systemic insulin resistance and AT inflammation due to macrophage-regulated autocrine and paracrine signaling that promotes anti-inflammatory responses in WAT (Fig. 2).
We have highlighted the role of type 2 immunity in metabolic disease. Recent studies have suggested that glucose intolerance and obesity induce the dysregulation of type 2 immunity. In addition, many studies have shown that type 2 immunity has a major influence on AT metabolism, including local effects, such as regulating AT browning, AT remodeling, and limiting excessive type 1 inflammatory responses, as well as systemic effects mediated via adipocytokines. Th2 cytokines, including IL-33, IL-4, and IL-13, have been identified as surrogate factors that stimulate the proliferation of M2 macrophages associated with low-grade inflammation in AT and differentiation of adipose precursors in metabolic homeostasis. Recently, we identified the roles of the Th2 cytokine (IL-4 and IL-13)-induced adipokine, GDF15, in AT in regulating systemic glucose metabolism via STAT6 activation. Moreover, we showed that mitochondrial OXPHOS is required to maintain these macrophage-regulating autocrine and paracrine signaling pathways via Th2 cytokine-induced secretion of GDF15. However, less is known regarding the mechanism of action of GDF15 in metabolic organs, such as muscle, liver, and AT. Interactive roles of Th2 cytokines and GDF15 must be further clarified by identification of the molecular action and signaling pathways of GDF15.
Figures and Tables
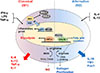 | Fig. 1Oxidative metabolism controls polarization of macrophages. Classically activated macrophages (M1) induce an aerobic glycolytic program. The hypoxia-inducible factor 1α (HIF1α) transcription factor also becomes activated and can drive production of proinflammatory cytokines. The key functional consequences are bacterial killing, mostly through the production of reactive oxygen species (ROS) and nitric oxide (NO) from L-arginine. Inflammatory genes are also activated by nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) activation and promoted by interferon γ (IFN-γ), lipopolysaccharide (LPS), and tumor necrosis factor-α (TNF-α). Alternative activated macrophages (M2) trigger a metabolic program including the electron transport chain as well as fatty acid oxidation, which is orchestrated by signal transducer and activator of transcription 6 (STAT6) and proliferator-activated receptor-γ coactivator 1β (PGC-1β). Arginase also drives the production of polyamines and L-ornithine. IRS, insulin receptor substrate; JNK, c-Jun N-terminal kinases; IKK, IκB kinase; AP1, activator protein 1; iNOS, nitric oxide synthase; IL, interleukin; YM1, chitinase-like 3. |
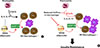 | Fig. 2Reduced oxidative capacity in macrophages triggers systemic insulin resistance characterized by classically activated macrophages (M1)-like polarization and adipose inflammation via T helper 2 (Th2) cytokine-growth differentiation factor 15 (GDF15) signaling. (A) Th2 cytokines, interleukin 4 (IL-4) and IL-13, alternative activated macrophages (M2)-like polarization and induced GDF15 secretion in white adipose tissue dependent on signal transducer and activator of transcription 6 (STAT6) activation. (B) Reduced oxidative capacity in macrophages impaired STAT6 activation and triggered M1-like polarization and lack of GDF15 secretion. Consequently, dysregulation of Th2 cytokine-GDF15 signaling due to dysfunction of mitochondria in adipose tissue triggers systemic insulin resistance. |
ACKNOWLEDGMENTS
This research was supported by Global Research Laboratory (GRL) Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT (No. NRF-2017K1A1A2013124).
References
1. Gause WC, Wynn TA, Allen JE. Type 2 immunity and wound healing: evolutionary refinement of adaptive immunity by helminths. Nat Rev Immunol. 2013; 13:607–614.
2. Odegaard JI, Chawla A. Type 2 responses at the interface between immunity and fat metabolism. Curr Opin Immunol. 2015; 36:67–72.
3. Paul WE, Zhu J. How are T(H)2-type immune responses initiated and amplified? Nat Rev Immunol. 2010; 10:225–235.
4. Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989; 7:145–173.
5. Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996; 383:787–793.
6. Wynn TA. Type 2 cytokines: mechanisms and therapeutic strategies. Nat Rev Immunol. 2015; 15:271–282.
7. Gieseck RL 3rd, Wilson MS, Wynn TA. Type 2 immunity in tissue repair and fibrosis. Nat Rev Immunol. 2018; 18:62–76.
8. Rosen ED, Spiegelman BM. What we talk about when we talk about fat. Cell. 2014; 156:20–44.
9. Saito M. Brown adipose tissue as a regulator of energy expenditure and body fat in humans. Diabetes Metab J. 2013; 37:22–29.
10. Mathis D. Immunological goings-on in visceral adipose tissue. Cell Metab. 2013; 17:851–859.
11. Osborn O, Olefsky JM. The cellular and signaling networks linking the immune system and metabolism in disease. Nat Med. 2012; 18:363–374.
12. Nakagami H. The mechanism of white and brown adipocyte differentiation. Diabetes Metab J. 2013; 37:85–90.
13. O'Sullivan TE, Rapp M, Fan X, Weizman OE, Bhardwaj P, Adams NM, Walzer T, Dannenberg AJ, Sun JC. Adipose-resident group 1 innate lymphoid cells promote obesity-associated insulin resistance. Immunity. 2016; 45:428–441.
14. Oldenhove G, Boucquey E, Taquin A, Acolty V, Bonetti L, Ryffel B, Le Bert M, Englebert K, Boon L, Moser M. PD-1 is involved in the dysregulation of type 2 innate lymphoid cells in a murine model of obesity. Cell Rep. 2018; 25:2053–2060.
15. Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, Nayer A, Lee J, Goldfine AB, Benoist C, Shoelson S, Mathis D. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med. 2009; 15:930–939.
16. Winer S, Chan Y, Paltser G, Truong D, Tsui H, Bahrami J, Dorfman R, Wang Y, Zielenski J, Mastronardi F, Maezawa Y, Drucker DJ, Engleman E, Winer D, Dosch HM. Normalization of obesity-associated insulin resistance through immunotherapy. Nat Med. 2009; 15:921–929.
17. Brestoff JR, Kim BS, Saenz SA, Stine RR, Monticelli LA, Sonnenberg GF, Thome JJ, Farber DL, Lutfy K, Seale P, Artis D. Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity. Nature. 2015; 519:242–246.
18. Wu D, Molofsky AB, Liang HE, Ricardo-Gonzalez RR, Jouihan HA, Bando JK, Chawla A, Locksley RM. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science. 2011; 332:243–247.
19. McNelis JC, Olefsky JM. Macrophages, immunity, and metabolic disease. Immunity. 2014; 41:36–48.
20. Benezech C, Jackson-Jones LH. ILC2 orchestration of local immune function in adipose tissue. Front Immunol. 2019; 10:171.
21. Vargovic P, Laukova M, Ukropec J, Manz G, Kvetnansky R. Prior repeated stress attenuates cold-induced immunomodulation associated with “browning” in mesenteric fat of rats. Cell Mol Neurobiol. 2018; 38:349–361.
22. Chung KJ, Nati M, Chavakis T, Chatzigeorgiou A. Innate immune cells in the adipose tissue. Rev Endocr Metab Disord. 2018; 19:283–292.
23. Mraz M, Haluzik M. The role of adipose tissue immune cells in obesity and low-grade inflammation. J Endocrinol. 2014; 222:R113–R127.
24. Cildir G, Akıncılar SC, Tergaonkar V. Chronic adipose tissue inflammation: all immune cells on the stage. Trends Mol Med. 2013; 19:487–500.
25. Molofsky AB, Nussbaum JC, Liang HE, Van Dyken SJ, Cheng LE, Mohapatra A, Chawla A, Locksley RM. Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages. J Exp Med. 2013; 210:535–549.
26. Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, Furusawa J, Ohtani M, Fujii H, Koyasu S. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010; 463:540–544.
27. Kraakman MJ, Murphy AJ, Jandeleit-Dahm K, Kammoun HL. Macrophage polarization in obesity and type 2 diabetes: weighing down our understanding of macrophage function? Front Immunol. 2014; 5:470.
28. Oh DY, Morinaga H, Talukdar S, Bae EJ, Olefsky JM. Increased macrophage migration into adipose tissue in obese mice. Diabetes. 2012; 61:346–354.
29. Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010; 72:219–246.
30. Anthony RM, Urban JF Jr, Alem F, Hamed HA, Rozo CT, Boucher JL, Van Rooijen N, Gause WC. Memory T(H)2 cells induce alternatively activated macrophages to mediate protection against nematode parasites. Nat Med. 2006; 12:955–960.
31. Porcheray F, Viaud S, Rimaniol AC, Léone C, Samah B, Dereuddre-Bosquet N, Dormont D, Gras G. Macrophage activation switching: an asset for the resolution of inflammation. Clin Exp Immunol. 2005; 142:481–489.
32. Vasanthakumar A, Moro K, Xin A, Liao Y, Gloury R, Kawamoto S, Fagarasan S, Mielke LA, Afshar-Sterle S, Masters SL, Nakae S, Saito H, Wentworth JM, Li P, Liao W, Leonard WJ, Smyth GK, Shi W, Nutt SL, Koyasu S, Kallies A. The transcriptional regulators IRF4, BATF and IL-33 orchestrate development and maintenance of adipose tissue-resident regulatory T cells. Nat Immunol. 2015; 16:276–285.
33. Sell H, Habich C, Eckel J. Adaptive immunity in obesity and insulin resistance. Nat Rev Endocrinol. 2012; 8:709–716.
34. Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007; 117:175–184.
35. Sun K, Kusminski CM, Scherer PE. Adipose tissue remodeling and obesity. J Clin Invest. 2011; 121:2094–2101.
36. Sun K, Tordjman J, Clement K, Scherer PE. Fibrosis and adipose tissue dysfunction. Cell Metab. 2013; 18:470–477.
37. Roberts J, Fallon PG, Hams E. The pivotal role of macrophages in metabolic distress. London: IntechOpen;2019. cited 2019 Sep 27. Available from: https://www.intechopen.com/online-first/the-pivotal-role-of-macrophages-in-metabolic-distress.
38. Pearce EL, Pearce EJ. Metabolic pathways in immune cell activation and quiescence. Immunity. 2013; 38:633–643.
39. O'Neill LA, Kishton RJ, Rathmell J. A guide to immunometabolism for immunologists. Nat Rev Immunol. 2016; 16:553–565.
40. Van den Bossche J, O'Neill LA, Menon D. Macrophage immunometabolism: where are we (going)? Trends Immunol. 2017; 38:395–406.
41. Van Dyken SJ, Locksley RM. Interleukin-4- and interleukin-13-mediated alternatively activated macrophages: roles in homeostasis and disease. Annu Rev Immunol. 2013; 31:317–343.
42. Stienstra R, Duval C, Keshtkar S, van der Laak J, Kersten S, Muller M. Peroxisome proliferator-activated receptor gamma activation promotes infiltration of alternatively activated macrophages into adipose tissue. J Biol Chem. 2008; 283:22620–22627.
43. Choe SS, Shin KC, Ka S, Lee YK, Chun JS, Kim JB. Macrophage HIF-2α ameliorates adipose tissue inflammation and insulin resistance in obesity. Diabetes. 2014; 63:3359–3371.
44. Fujisaka S, Usui I, Ikutani M, Aminuddin A, Takikawa A, Tsuneyama K, Mahmood A, Goda N, Nagai Y, Takatsu K, Tobe K. Adipose tissue hypoxia induces inflammatory M1 polarity of macrophages in an HIF-1α-dependent and HIF-1α-independent manner in obese mice. Diabetologia. 2013; 56:1403–1412.
45. Rodriguez-Prados JC, Traves PG, Cuenca J, Rico D, Aragones J, Martin-Sanz P, Cascante M, Bosca L. Substrate fate in activated macrophages: a comparison between innate, classic, and alternative activation. J Immunol. 2010; 185:605–614.
46. Odegaard JI, Ricardo-Gonzalez RR, Red Eagle A, Vats D, Morel CR, Goforth MH, Subramanian V, Mukundan L, Ferrante AW, Chawla A. Alternative M2 activation of Kupffer cells by PPARdelta ameliorates obesity-induced insulin resistance. Cell Metab. 2008; 7:496–507.
47. Dalmas E, Toubal A, Alzaid F, Blazek K, Eames HL, Lebozec K, Pini M, Hainault I, Montastier E, Denis RG, Ancel P, Lacombe A, Ling Y, Allatif O, Cruciani-Guglielmacci C, Andre S, Viguerie N, Poitou C, Stich V, Torcivia A, Foufelle F, Luquet S, Aron-Wisnewsky J, Langin D, Clement K, Udalova IA, Venteclef N. Irf5 deficiency in macrophages promotes beneficial adipose tissue expansion and insulin sensitivity during obesity. Nat Med. 2015; 21:610–618.
48. Spits H, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, Koyasu S, Locksley RM, McKenzie AN, Mebius RE, Powrie F, Vivier E. Innate lymphoid cells: a proposal for uniform nomenclature. Nat Rev Immunol. 2013; 13:145–149.
49. Walker JA, McKenzie AN. Development and function of group 2 innate lymphoid cells. Curr Opin Immunol. 2013; 25:148–155.
50. Monticelli LA, Osborne LC, Noti M, Tran SV, Zaiss DM, Artis D. IL-33 promotes an innate immune pathway of intestinal tissue protection dependent on amphiregulin-EGFR interactions. Proc Natl Acad Sci U S A. 2015; 112:10762–10767.
51. Walker JA, Barlow JL, McKenzie AN. Innate lymphoid cells: how did we miss them? Nat Rev Immunol. 2013; 13:75–87.
52. Kolodin D, van Panhuys N, Li C, Magnuson AM, Cipolletta D, Miller CM, Wagers A, Germain RN, Benoist C, Mathis D. Antigen- and cytokine-driven accumulation of regulatory T cells in visceral adipose tissue of lean mice. Cell Metab. 2015; 21:543–557.
53. Nussbaum JC, Van Dyken SJ, von Moltke J, Cheng LE, Mohapatra A, Molofsky AB, Thornton EE, Krummel MF, Chawla A, Liang HE, Locksley RM. Type 2 innate lymphoid cells control eosinophil homeostasis. Nature. 2013; 502:245–248.
54. Stein M, Keshav S, Harris N, Gordon S. Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J Exp Med. 1992; 176:287–292.
55. Ricardo-Gonzalez RR, Red Eagle A, Odegaard JI, Jouihan H, Morel CR, Heredia JE, Mukundan L, Wu D, Locksley RM, Chawla A. IL-4/STAT6 immune axis regulates peripheral nutrient metabolism and insulin sensitivity. Proc Natl Acad Sci U S A. 2010; 107:22617–22622.
56. Stanya KJ, Jacobi D, Liu S, Bhargava P, Dai L, Gangl MR, Inouye K, Barlow JL, Ji Y, Mizgerd JP, Qi L, Shi H, McKenzie AN, Lee CH. Direct control of hepatic glucose production by interleukin-13 in mice. J Clin Invest. 2013; 123:261–271.
57. Hams E, Locksley RM, McKenzie AN, Fallon PG. Cutting edge: IL-25 elicitsf innate lymphoid type 2 and type II NKT cells that regulate obesity in mice. J Immunol. 2013; 191:5349–5353.
58. Rao RR, Long JZ, White JP, Svensson KJ, Lou J, Lokurkar I, Jedrychowski MP, Ruas JL, Wrann CD, Lo JC, Camera DM, Lachey J, Gygi S, Seehra J, Hawley JA, Spiegelman BM. Meteorin-like is a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis. Cell. 2014; 157:1279–1291.
59. Qiu Y, Nguyen KD, Odegaard JI, Cui X, Tian X, Locksley RM, Palmiter RD, Chawla A. Eosinophils and type 2 cytokine signaling in macrophages orchestrate development of functional beige fat. Cell. 2014; 157:1292–1308.
60. Cipolletta D, Feuerer M, Li A, Kamei N, Lee J, Shoelson SE, Benoist C, Mathis D. PPAR-γ is a major driver of the accumulation and phenotype of adipose tissue Treg cells. Nature. 2012; 486:549–553.
61. Han JM, Wu D, Denroche HC, Yao Y, Verchere CB, Levings MK. IL-33 reverses an obesity-induced deficit in visceral adipose tissue ST2+ T regulatory cells and ameliorates adipose tissue inflammation and insulin resistance. J Immunol. 2015; 194:4777–4783.
62. Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circ Res. 2005; 96:939–949.
63. Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, Otsu M, Hara K, Ueki K, Sugiura S, Yoshimura K, Kadowaki T, Nagai R. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009; 15:914–920.
64. Tilg H, Moschen AR. Inflammatory mechanisms in the regulation of insulin resistance. Mol Med. 2008; 14:222–231.
65. Fain JN, Madan AK, Hiler ML, Cheema P, Bahouth SW. Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology. 2004; 145:2273–2282.
66. Csoka B, Koscso B, Toro G, Kokai E, Virag L, Nemeth ZH, Pacher P, Bai P, Hasko G. A2B adenosine receptors prevent insulin resistance by inhibiting adipose tissue inflammation via maintaining alternative macrophage activation. Diabetes. 2014; 63:850–866.
67. Kang K, Reilly SM, Karabacak V, Gangl MR, Fitzgerald K, Hatano B, Lee CH. Adipocyte-derived Th2 cytokines and myeloid PPARdelta regulate macrophage polarization and insulin sensitivity. Cell Metab. 2008; 7:485–495.
68. Liao X, Sharma N, Kapadia F, Zhou G, Lu Y, Hong H, Paruchuri K, Mahabeleshwar GH, Dalmas E, Venteclef N, Flask CA, Kim J, Doreian BW, Lu KQ, Kaestner KH, Hamik A, Clement K, Jain MK. Krüppel-like factor 4 regulates macrophage polarization. J Clin Invest. 2011; 121:2736–2749.
69. Bolus WR, Peterson KR, Hubler MJ, Kennedy AJ, Gruen ML, Hasty AH. Elevating adipose eosinophils in obese mice to physiologically normal levels does not rescue metabolic impairments. Mol Metab. 2018; 8:86–95.
70. Lee MW, Odegaard JI, Mukundan L, Qiu Y, Molofsky AB, Nussbaum JC, Yun K, Locksley RM, Chawla A. Activated type 2 innate lymphoid cells regulate beige fat biogenesis. Cell. 2015; 160:74–87.
71. Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011; 11:85–97.
72. Friedman J. The long road to leptin. J Clin Invest. 2016; 126:4727–4734.
73. Shuldiner AR, Yang R, Gong DW. Resistin, obesity, and insulin resistance: the emerging role of the adipocyte as an endocrine organ. N Engl J Med. 2001; 345:1345–1346.
74. Timper K, Denson JL, Steculorum SM, Heilinger C, Engstrom-Ruud L, Wunderlich CM, Rose-John S, Wunderlich FT, Bruning JC. IL-6 improves energy and glucose homeostasis in obesity via enhanced central IL-6 trans-signaling. Cell Rep. 2017; 19:267–280.
75. Itoh N. FGF21 as a hepatokine, adipokine, and myokine in metabolism and diseases. Front Endocrinol (Lausanne). 2014; 5:107.
76. Shin MY, Kim JM, Kang YE, Kim MK, Joung KH, Lee JH, Kim KS, Kim HJ, Ku BJ, Shong M. Association between growth differentiation factor 15 (GDF15) and cardiovascular risk in patients with newly diagnosed type 2 diabetes mellitus. J Korean Med Sci. 2016; 31:1413–1418.
77. Kang YE, Kim JM, Choung S, Joung KH, Lee JH, Kim HJ, Ku BJ. Comparison of serum neuregulin 4 (Nrg4) levels in adults with newly diagnosed type 2 diabetes mellitus and controls without diabetes. Diabetes Res Clin Pract. 2016; 117:1–3.
78. Kang YE, Kim JM, Joung KH, Lee JH, You BR, Choi MJ, Ryu MJ, Ko YB, Lee MA, Lee J, Ku BJ, Shong M, Lee KH, Kim HJ. The roles of adipokines, proinflammatory cytokines, and adipose tissue macrophages in obesity-associated insulin resistance in modest obesity and early metabolic dysfunction. PLoS One. 2016; 11:e0154003.
79. Bouhlel MA, Derudas B, Rigamonti E, Dievart R, Brozek J, Haulon S, Zawadzki C, Jude B, Torpier G, Marx N, Staels B, Chinetti-Gbaguidi G. PPARgamma activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metab. 2007; 6:137–143.
80. Chawla A. Control of macrophage activation and function by PPARs. Circ Res. 2010; 106:1559–1569.
81. Lee SE, Kang SG, Choi MJ, Jung SB, Ryu MJ, Chung HK, Chang JY, Kim YK, Lee JH, Kim KS, Kim HJ, Lee HK, Yi HS, Shong M. Growth differentiation factor 15 mediates systemic glucose regulatory action of T-Helper type 2 cytokines. Diabetes. 2017; 66:2774–2788.
82. Emmerson PJ, Wang F, Du Y, Liu Q, Pickard RT, Gonciarz MD, Coskun T, Hamang MJ, Sindelar DK, Ballman KK, Foltz LA, Muppidi A, Alsina-Fernandez J, Barnard GC, Tang JX, Liu X, Mao X, Siegel R, Sloan JH, Mitchell PJ, Zhang BB, Gimeno RE, Shan B, Wu X. The metabolic effects of GDF15 are mediated by the orphan receptor GFRAL. Nat Med. 2017; 23:1215–1219.
83. Yang L, Chang CC, Sun Z, Madsen D, Zhu H, Padkjær SB, Wu X, Huang T, Hultman K, Paulsen SJ, Wang J, Bugge A, Frantzen JB, Norgaard P, Jeppesen JF, Yang Z, Secher A, Chen H, Li X, John LM, Shan B, He Z, Gao X, Su J, Hansen KT, Yang W, Jorgensen SB. GFRAL is the receptor for GDF15 and is required for the anti-obesity effects of the ligand. Nat Med. 2017; 23:1158–1166.
84. Ding Q, Mracek T, Gonzalez-Muniesa P, Kos K, Wilding J, Trayhurn P, Bing C. Identification of macrophage inhibitory cytokine-1 in adipose tissue and its secretion as an adipokine by human adipocytes. Endocrinology. 2009; 150:1688–1696.
85. Chung HK, Ryu D, Kim KS, Chang JY, Kim YK, Yi HS, Kang SG, Choi MJ, Lee SE, Jung SB, Ryu MJ, Kim SJ, Kweon GR, Kim H, Hwang JH, Lee CH, Lee SJ, Wall CE, Downes M, Evans RM, Auwerx J, Shong M. Growth differentiation factor 15 is a myomitokine governing systemic energy homeostasis. J Cell Biol. 2017; 216:149–165.
86. Hong JH, Chung HK, Park HY, Joung KH, Lee JH, Jung JG, Kim KS, Kim HJ, Ku BJ, Shong M. GDF15 is a novel biomarker for impaired fasting glucose. Diabetes Metab J. 2014; 38:472–479.
87. Chung HK, Kim JT, Kim HW, Kwon M, Kim SY, Shong M, Kim KS, Yi HS. GDF15 deficiency exacerbates chronic alcohol- and carbon tetrachloride-induced liver injury. Sci Rep. 2017; 7:17238.
88. West AP, Brodsky IE, Rahner C, Woo DK, Erdjument-Bromage H, Tempst P, Walsh MC, Choi Y, Shadel GS, Ghosh S. TLR signalling augments macrophage bactericidal activity through mitochondrial ROS. Nature. 2011; 472:476–480.
89. Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, Morel CR, Subramanian V, Mukundan L, Red Eagle A, Vats D, Brombacher F, Ferrante AW, Chawla A. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature. 2007; 447:1116–1120.
90. Huang SC, Everts B, Ivanova Y, O'Sullivan D, Nascimento M, Smith AM, Beatty W, Love-Gregory L, Lam WY, O'Neill CM, Yan C, Du H, Abumrad NA, Urban JF Jr, Artyomov MN, Pearce EL, Pearce EJ. Cell-intrinsic lysosomal lipolysis is essential for alternative activation of macrophages. Nat Immunol. 2014; 15:846–855.
91. Jha AK, Huang SC, Sergushichev A, Lampropoulou V, Ivanova Y, Loginicheva E, Chmielewski K, Stewart KM, Ashall J, Everts B, Pearce EJ, Driggers EM, Artyomov MN. Network integration of parallel metabolic and transcriptional data reveals metabolic modules that regulate macrophage polarization. Immunity. 2015; 42:419–430.
92. Jung SB, Choi MJ, Ryu D, Yi HS, Lee SE, Chang JY, Chung HK, Kim YK, Kang SG, Lee JH, Kim KS, Kim HJ, Kim CS, Lee CH, Williams RW, Kim H, Lee HK, Auwerx J, Shong M. Reduced oxidative capacity in macrophages results in systemic insulin resistance. Nat Commun. 2018; 9:1551.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download