1. Tesfaye S, Boulton AJ, Dyck PJ, Freeman R, Horowitz M, Kempler P, Lauria G, Malik RA, Spallone V, Vinik A, Bernardi L, Valensi P. Toronto Diabetic Neuropathy Expert Group. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 2010; 33:2285–2293. PMID:
20876709.

2. Spallone V, Ziegler D, Freeman R, Bernardi L, Frontoni S, Pop-Busui R, Stevens M, Kempler P, Hilsted J, Tesfaye S, Low P, Valensi P. Toronto Consensus Panel on Diabetic Neuropathy. Cardiovascular autonomic neuropathy in diabetes: clinical impact, assessment, diagnosis, and management. Diabetes Metab Res Rev. 2011; 27:639–653. PMID:
21695768.

3. Bernardi L, Spallone V, Stevens M, Hilsted J, Frontoni S, Pop-Busui R, Ziegler D, Kempler P, Freeman R, Low P, Tesfaye S, Valensi P. Toronto Consensus Panel on Diabetic Neuropathy. Methods of investigation for cardiac autonomic dysfunction in human research studies. Diabetes Metab Res Rev. 2011; 27:654–664. PMID:
21695761.

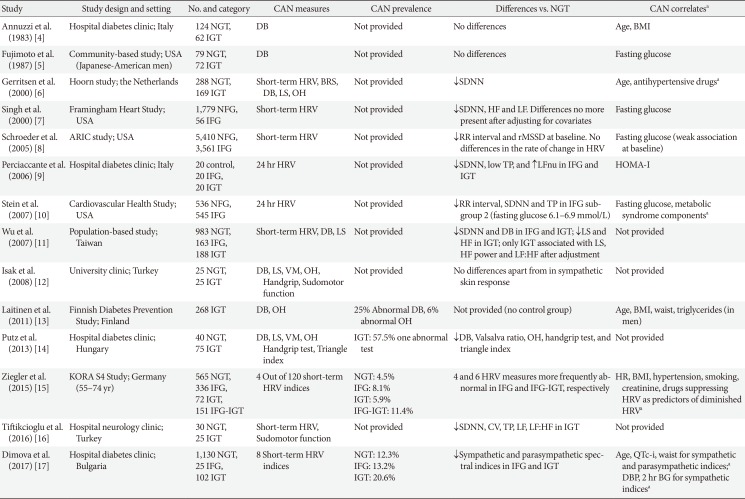
4. Annuzzi G, Rivellese A, Vaccaro O, Ferrante MR, Riccardi G, Mancini M. The relationship between blood glucose concentration and beat-to-beat variation in asymptomatic subjects. Acta Diabetol Lat. 1983; 20:57–62. PMID:
6858543.

5. Fujimoto WY, Leonetti DL, Kinyoun JL, Shuman WP, Stolov WC, Wahl PW. Prevalence of complications among second-generation Japanese-American men with diabetes, impaired glucose tolerance, or normal glucose tolerance. Diabetes. 1987; 36:730–739. PMID:
3569672.

6. Gerritsen J, Dekker JM, TenVoorde BJ, Bertelsmann FW, Kostense PJ, Stehouwer CD, Heine RJ, Nijpels G, Heethaar RM, Bouter LM. Glucose tolerance and other determinants of cardiovascular autonomic function: the Hoorn Study. Diabetologia. 2000; 43:561–570. PMID:
10855530.

7. Singh JP, Larson MG, O'Donnell CJ, Wilson PF, Tsuji H, Lloyd-Jones DM, Levy D. Association of hyperglycemia with reduced heart rate variability (The Framingham Heart Study). Am J Cardiol. 2000; 86:309–312. PMID:
10922439.

8. Schroeder EB, Chambless LE, Liao D, Prineas RJ, Evans GW, Rosamond WD, Heiss G. Atherosclerosis Risk in Communities (ARIC) study. Diabetes, glucose, insulin, and heart rate variability: the Atherosclerosis Risk in Communities (ARIC) study. Diabetes Care. 2005; 28:668–674. PMID:
15735206.
9. Perciaccante A, Fiorentini A, Paris A, Serra P, Tubani L. Circadian rhythm of the autonomic nervous system in insulin resistant subjects with normoglycemia, impaired fasting glycemia, impaired glucose tolerance, type 2 diabetes mellitus. BMC Cardiovasc Disord. 2006; 6:19. PMID:
16670002.

10. Stein PK, Barzilay JI, Domitrovich PP, Chaves PM, Gottdiener JS, Heckbert SR, Kronmal RA. The relationship of heart rate and heart rate variability to non-diabetic fasting glucose levels and the metabolic syndrome: the Cardiovascular Health Study. Diabet Med. 2007; 24:855–863. PMID:
17403115.

11. Wu JS, Yang YC, Lin TS, Huang YH, Chen JJ, Lu FH, Wu CH, Chang CJ. Epidemiological evidence of altered cardiac autonomic function in subjects with impaired glucose tolerance but not isolated impaired fasting glucose. J Clin Endocrinol Metab. 2007; 92:3885–3889. PMID:
17666483.

12. Isak B, Oflazoglu B, Tanridag T, Yitmen I, Us O. Evaluation of peripheral and autonomic neuropathy among patients with newly diagnosed impaired glucose tolerance. Diabetes Metab Res Rev. 2008; 24:563–569. PMID:
18636432.

13. Laitinen T, Lindstrom J, Eriksson J, Ilanne-Parikka P, Aunola S, Keinanen-Kiukaanniemi S, Tuomilehto J, Uusitupa M. Cardiovascular autonomic dysfunction is associated with central obesity in persons with impaired glucose tolerance. Diabet Med. 2011; 28:699–704. PMID:
21388444.

14. Putz Z, Nemeth N, Istenes I, Martos T, Gandhi RA, Korei AE, Hermanyi Z, Szathmari M, Jermendy G, Tesfaye S, Tabak AG, Kempler P. Autonomic dysfunction and circadian blood pressure variations in people with impaired glucose tolerance. Diabet Med. 2013; 30:358–362. PMID:
23278478.

15. Ziegler D, Voss A, Rathmann W, Strom A, Perz S, Roden M, Peters A, Meisinger C. KORA Study Group. Increased prevalence of cardiac autonomic dysfunction at different degrees of glucose intolerance in the general population: the KORA S4 survey. Diabetologia. 2015; 58:1118–1128. PMID:
25724570.

16. Tiftikcioglu BI, Bilgin S, Duksal T, Kose S, Zorlu Y. Autonomic neuropathy and endothelial dysfunction in patients with impaired glucose tolerance or type 2 diabetes mellitus. Medicine (Baltimore). 2016; 95:e3340. PMID:
27057914.

17. Dimova R, Tankova T, Guergueltcheva V, Tournev I, Chakarova N, Grozeva G, Dakovska L. Risk factors for autonomic and somatic nerve dysfunction in different stages of glucose tolerance. J Diabetes Complications. 2017; 31:537–543. PMID:
27894750.

18. Ribeiro MJ, Sacramento JF, Gonzalez C, Guarino MP, Monteiro EC, Conde SV. Carotid body denervation prevents the development of insulin resistance and hypertension induced by hypercaloric diets. Diabetes. 2013; 62:2905–2916. PMID:
23530003.

19. Greco C, Spallone V. Obstructive sleep apnoea syndrome and diabetes. Fortuitous association or interaction? Curr Diabetes Rev. 2015; 12:129–155. PMID:
25808418.

20. Ziegler D, Strom A, Kupriyanova Y, Bierwagen A, Bonhof GJ, Bodis K, Mussig K, Szendroedi J, Bobrov P, Markgraf DF, Hwang JH, Roden M. GDS Group. Association of lower cardiovagal tone and baroreflex sensitivity with higher liver fat content early in type 2 diabetes. J Clin Endocrinol Metab. 2018; 103:1130–1138. PMID:
29267946.

21. Herder C, Schamarek I, Nowotny B, Carstensen-Kirberg M, Straßburger K, Nowotny P, Kannenberg JM, Strom A, Puttgen S, Mussig K, Szendroedi J, Roden M, Ziegler D. German Diabetes Study Group. Inflammatory markers are associated with cardiac autonomic dysfunction in recent-onset type 2 diabetes. Heart. 2017; 103:63–70. PMID:
27481890.

22. Hansen CS, Vistisen D, Jorgensen ME, Witte DR, Brunner EJ, Tabak AG, Kivimaki M, Roden M, Malik M, Herder C. Adiponectin, biomarkers of inflammation and changes in cardiac autonomic function: Whitehall II study. Cardiovasc Diabetol. 2017; 16:153. PMID:
29195493.

23. Abboud FM, Singh MV. Autonomic regulation of the immune system in cardiovascular diseases. Adv Physiol Educ. 2017; 41:578–593. PMID:
29138216.

24. Guarino D, Nannipieri M, Iervasi G, Taddei S, Bruno RM. The role of the autonomic nervous system in the pathophysiology of obesity. Front Physiol. 2017; 8:665. PMID:
28966594.

25. Wulsin LR, Horn PS, Perry JL, Massaro JM, D'Agostino RB. Autonomic imbalance as a predictor of metabolic risks, cardiovascular disease, diabetes, and mortality. J Clin Endocrinol Metab. 2015; 100:2443–2448. PMID:
26047073.

26. Zoppini G, Cacciatori V, Raimondo D, Gemma M, Trombetta M, Dauriz M, Brangani C, Pichiri I, Negri C, Stoico V, Bergamini C, Targher G, Santi L, Thomaseth K, Bellavere F, Bonadonna RC, Bonora E. Prevalence of cardiovascular autonomic neuropathy in a cohort of patients with newly diagnosed type 2 diabetes: the Verona Newly Diagnosed Type 2 Diabetes Study (VNDS). Diabetes Care. 2015; 38:1487–1493. PMID:
26068862.

27. Andersen ST, Witte DR, Fleischer J, Andersen H, Lauritzen T, Jorgensen ME, Jensen TS, Pop-Busui R, Charles M. Risk factors for the presence and progression of cardiovascular autonomic neuropathy in type 2 diabetes: ADDITION-Denmark. Diabetes Care. 2018; 41:2586–2594. PMID:
30305347.

28. Martin CL, Albers JW, Pop-Busui R. DCCT/EDIC Research Group. Neuropathy and related findings in the diabetes control and complications trial/epidemiology of diabetes interventions and complications study. Diabetes Care. 2014; 37:31–38. PMID:
24356595.

29. Fleischer J, Yderstraede K, Gulichsen E, Jakobsen PE, Lervang HH, Eldrup E, Nygaard H, Tarnow L, Ejskjaer N. Cardiovascular autonomic neuropathy is associated with macrovascular risk factors in type 2 diabetes: new technology used for routine large-scale screening adds new insight. J Diabetes Sci Technol. 2014; 8:874–880. PMID:
24876410.
30. Tannus LR, Drummond KR, Clemente EL, da Matta Mde F, Gomes MB. Brazilian Type 1 Diabetes Study Group (BrazDiab1SG). Predictors of cardiovascular autonomic neuropathy in patients with type 1 diabetes. Front Endocrinol (Lausanne). 2014; 5:191. PMID:
25505446.

31. Hansen CS, Jensen JS, Ridderstrale M, Vistisen D, Jorgensen ME, Fleischer J. Vitamin B12 deficiency is associated with cardiovascular autonomic neuropathy in patients with type 2 diabetes. J Diabetes Complications. 2017; 31:202–208. PMID:
27638143.

32. Maser RE, Lenhard MJ, Pohlig RT. Vitamin D insufficiency is associated with reduced parasympathetic nerve fiber function in type 2 diabetes. Endocr Pract. 2015; 21:174–181. PMID:
25297669.

33. Jung CH, Jung SH, Kim KJ, Kim BY, Kim CH, Kang SK, Mok JO. The relationship between vitamin D status and cardiac autonomic neuropathy in patients with type 2 diabetes mellitus. Diab Vasc Dis Res. 2015; 12:342–351. PMID:
26150192.

34. Hansen CS, Fleischer J, Vistisen D, Ridderstrale M, Jensen JS, Jorgensen ME. High and low vitamin D level is associated with cardiovascular autonomic neuropathy in people with type 1 and type 2 diabetes. Diabet Med. 2017; 34:364–371. PMID:
27696502.

35. Chung JO, Cho DH, Chung DJ, Chung MY. Physiological serum bilirubin concentrations are inversely associated with the prevalence of cardiovascular autonomic neuropathy in patients with type 2 diabetes. Diabet Med. 2014; 31:185–191. PMID:
24147832.

36. Ziegler D, Buchholz S, Sohr C, Nourooz-Zadeh J, Roden M. Oxidative stress predicts progression of peripheral and cardiac autonomic nerve dysfunction over 6 years in diabetic patients. Acta Diabetol. 2015; 52:65–72. PMID:
24898524.
37. Gupta R, Misra A. Epidemiology of microvascular complications of diabetes in South Asians and comparison with other ethnicities. J Diabetes. 2016; 8:470–482. PMID:
26781344.

38. Abbott CA, Chaturvedi N, Malik RA, Salgami E, Yates AP, Pemberton PW, Boulton AJ. Explanations for the lower rates of diabetic neuropathy in Indian Asians versus Europeans. Diabetes Care. 2010; 33:1325–1330. PMID:
20215455.

39. Tahrani AA, Altaf QA, Piya MK, Barnett AH. Peripheral and autonomic neuropathy in south Asians and white Caucasians with type 2 diabetes mellitus: possible explanations for epidemiological differences. J Diabetes Res. 2017; 2017:1273789. PMID:
28409160.

40. Ciccacci C, Morganti R, Di Fusco D, D'Amato C, Cacciotti L, Greco C, Rufini S, Novelli G, Sangiuolo F, Marfia GA, Borgiani P, Spallone V. Common polymorphisms in MIR146a, MIR128a and MIR27a genes contribute to neuropathy susceptibility in type 2 diabetes. Acta Diabetol. 2014; 51:663–671. PMID:
24682535.

41. Ciccacci C, Latini A, Greco C, Politi C, D'Amato C, Lauro D, Novelli G, Borgiani P, Spallone V. Association between a MIR499A polymorphism and diabetic neuropathy in type 2 diabetes. J Diabetes Complications. 2018; 32:11–17. PMID:
29108839.

42. Siegelaar SE, Kilpatrick ES, Rigby AS, Atkin SL, Hoekstra JB, Devries JH. Glucose variability does not contribute to the development of peripheral and autonomic neuropathy in type 1 diabetes: data from the DCCT. Diabetologia. 2009; 52:2229–2232. PMID:
19672575.

43. Lachin JM, Bebu I, Bergenstal RM, Pop-Busui R, Service FJ, Zinman B, Nathan DM. DCCT/EDIC Research Group. Association of glycemic variability in type 1 diabetes with progression of microvascular outcomes in the diabetes control and complications trial. Diabetes Care. 2017; 40:777–783. PMID:
28404658.

44. Nyiraty S, Pesei F, Orosz A, Coluzzi S, Vagi OE, Lengyel C, Abraham G, Frontoni S, Kempler P, Varkonyi T. Cardiovascular autonomic neuropathy and glucose variability in patients with type 1 diabetes: is there an association? Front Endocrinol (Lausanne). 2018; 9:174. PMID:
29725320.

45. Di Flaviani A, Picconi F, Di Stefano P, Giordani I, Malandrucco I, Maggio P, Palazzo P, Sgreccia F, Peraldo C, Farina F, Frajese G, Frontoni S. Impact of glycemic and blood pressure variability on surrogate measures of cardiovascular outcomes in type 2 diabetic patients. Diabetes Care. 2011; 34:1605–1609. PMID:
21610126.

46. Kalopita S, Liatis S, Thomakos P, Vlahodimitris I, Stathi C, Katsilambros N, Tentolouris N, Makrilakis K. Relationship between autonomic nervous system function and continuous interstitial glucose measurement in patients with type 2 diabetes. J Diabetes Res. 2014; 2014:835392. PMID:
25165724.

47. Jun JE, Jin SM, Baek J, Oh S, Hur KY, Lee MS, Lee MK, Kim JH. The association between glycemic variability and diabetic cardiovascular autonomic neuropathy in patients with type 2 diabetes. Cardiovasc Diabetol. 2015; 14:70. PMID:
26041130.

48. Xu W, Zhu Y, Yang X, Deng H, Yan J, Lin S, Yang H, Chen H, Weng J. Glycemic variability is an important risk factor for cardiovascular autonomic neuropathy in newly diagnosed type 2 diabetic patients. Int J Cardiol. 2016; 215:263–268. PMID:
27128543.

49. Fleischer J, Lebech Cichosz S, Hoeyem P, Laugesen E, Loegstrup Poulsen P, Sandahl Christiansen J, Tarnow L, Hansen TK. Glycemic variability is associated with reduced cardiac autonomic modulation in women with type 2 diabetes. Diabetes Care. 2015; 38:682–688. PMID:
25573884.

50. Klimontov VV, Myakina NE, Tyan NV. Heart rate variability is associated with interstitial glucose fluctuations in type 2 diabetic women treated with insulin. Springerplus. 2016; 5:337. PMID:
27066358.

51. Fleischer J, Laugesen E, Cichosz SL, Hoeyem P, Dejgaard TF, Poulsen PL, Tarnow L, Hansen TK. Continuous glucose monitoring adds information beyond HbA1c in well-controlled diabetes patients with early cardiovascular autonomic neuropathy. J Diabetes Complications. 2017; 31:1389–1393. PMID:
28728915.

52. Vinik AI, Ziegler D. Diabetic cardiovascular autonomic neuropathy. Circulation. 2007; 115:387–397. PMID:
17242296.

53. Soedamah-Muthu SS, Chaturvedi N, Witte DR, Stevens LK, Porta M, Fuller JH. EURODIAB Prospective Complications Study Group. Relationship between risk factors and mortality in type 1 diabetic patients in Europe: the EURODIAB Prospective Complications Study (PCS). Diabetes Care. 2008; 31:1360–1366. PMID:
18375412.

54. Pop-Busui R, Evans GW, Gerstein HC, Fonseca V, Fleg JL, Hoogwerf BJ, Genuth S, Grimm RH, Corson MA, Prineas R. Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of cardiac autonomic dysfunction on mortality risk in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Diabetes Care. 2010; 33:1578–1584. PMID:
20215456.

55. Young LH, Wackers FJ, Chyun DA, Davey JA, Barrett EJ, Taillefer R, Heller GV, Iskandrian AE, Wittlin SD, Filipchuk N, Ratner RE, Inzucchi SE. DIAD Investigators. Cardiac outcomes after screening for asymptomatic coronary artery disease in patients with type 2 diabetes: the DIAD study: a randomized controlled trial. JAMA. 2009; 301:1547–1555. PMID:
19366774.
56. Cha SA, Yun JS, Lim TS, Min K, Song KH, Yoo KD, Park YM, Ahn YB, Ko SH. Diabetic cardiovascular autonomic neuropathy predicts recurrent cardiovascular diseases in patients with type 2 diabetes. PLoS One. 2016; 11:e0164807. PMID:
27741306.

57. Pop-Busui R, Braffett BH, Zinman B, Martin C, White NH, Herman WH, Genuth S, Gubitosi-Klug R. DCCT/EDIC Research Group. Cardiovascular autonomic neuropathy and cardiovascular outcomes in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) study. Diabetes Care. 2017; 40:94–100. PMID:
27803120.

58. Yun JS, Kim JH, Song KH, Ahn YB, Yoon KH, Yoo KD, Park YM, Ko SH. Cardiovascular autonomic dysfunction predicts severe hypoglycemia in patients with type 2 diabetes: a 10-year follow-up study. Diabetes Care. 2014; 37:235–241. PMID:
23959567.

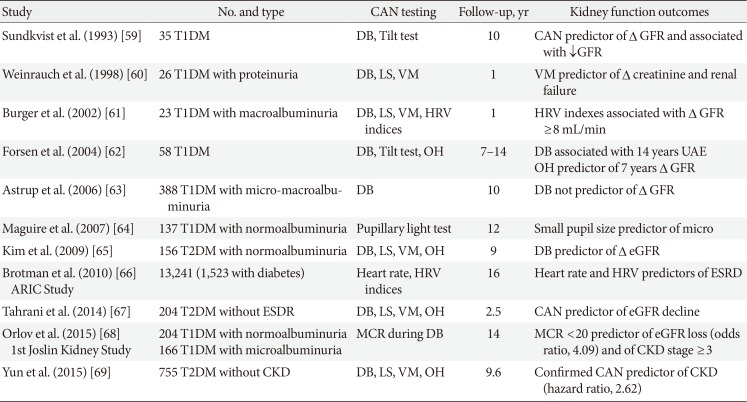
59. Sundkvist G, Lilja B. Autonomic neuropathy predicts deterioration in glomerular filtration rate in patients with IDDM. Diabetes Care. 1993; 16:773–779. PMID:
8495619.

60. Weinrauch LA, Kennedy FP, Gleason RE, Keough J, D'Elia JA. Relationship between autonomic function and progression of renal disease in diabetic proteinuria: clinical correlations and implications for blood pressure control. Am J Hypertens. 1998; 11:302–308. PMID:
9544870.

61. Burger AJ, D'Elia JA, Weinrauch LA, Lerman I, Gaur A. Marked abnormalities in heart rate variability are associated with progressive deterioration of renal function in type I diabetic patients with overt nephropathy. Int J Cardiol. 2002; 86:281–287. PMID:
12419567.

62. Forsen A, Kangro M, Sterner G, Norrgren K, Thorsson O, Wollmer P, Sundkvist G. A 14-year prospective study of autonomic nerve function in type 1 diabetic patients: association with nephropathy. Diabet Med. 2004; 21:852–858. PMID:
15270788.

63. Astrup AS, Tarnow L, Rossing P, Hansen BV, Hilsted J, Parving HH. Cardiac autonomic neuropathy predicts cardiovascular morbidity and mortality in type 1 diabetic patients with diabetic nephropathy. Diabetes Care. 2006; 29:334–339. PMID:
16443883.

64. Maguire AM, Craig ME, Craighead A, Chan AK, Cusumano JM, Hing SJ, Silink M, Howard NJ, Donaghue KC. Autonomic nerve testing predicts the development of complications: a 12-year follow-up study. Diabetes Care. 2007; 30:77–82. PMID:
17192337.
65. Kim YK, Lee JE, Kim YG, Kim DJ, Oh HY, Yang CW, Kim KW, Huh W. Cardiac autonomic neuropathy as a predictor of deterioration of the renal function in normoalbuminuric, normotensive patients with type 2 diabetes mellitus. J Korean Med Sci. 2009; 24(Suppl):S69–S74. PMID:
19194565.

66. Brotman DJ, Bash LD, Qayyum R, Crews D, Whitsel EA, Astor BC, Coresh J. Heart rate variability predicts ESRD and CKD-related hospitalization. J Am Soc Nephrol. 2010; 21:1560–1570. PMID:
20616169.

67. Tahrani AA, Dubb K, Raymond NT, Begum S, Altaf QA, Sadiqi H, Piya MK, Stevens MJ. Cardiac autonomic neuropathy predicts renal function decline in patients with type 2 diabetes: a cohort study. Diabetologia. 2014; 57:1249–1256. PMID:
24623102.

68. Orlov S, Cherney DZ, Pop-Busui R, Lovblom LE, Ficociello LH, Smiles AM, Warram JH, Krolewski AS, Perkins BA. Cardiac autonomic neuropathy and early progressive renal decline in patients with nonmacroalbuminuric type 1 diabetes. Clin J Am Soc Nephrol. 2015; 10:1136–1144. PMID:
26092828.

69. Yun JS, Ahn YB, Song KH, Yoo KD, Kim HW, Park YM, Ko SH. The association between abnormal heart rate variability and new onset of chronic kidney disease in patients with type 2 diabetes: a ten-year follow-up study. Diabetes Res Clin Pract. 2015; 108:31–37. PMID:
25656759.

70. Spallone V, Maiello MR, Kurukulasuriya N, Barini A, Lovecchio M, Tartaglione R, Mennuni G, Menzinger G. Does autonomic neuropathy play a role in erythropoietin regulation in non-proteinuric type 2 diabetic patients? Diabet Med. 2004; 21:1174–1180. PMID:
15498082.

71. Aune D, Sen A, o'Hartaigh B, Janszky I, Romundstad PR, Tonstad S, Vatten LJ. Resting heart rate and the risk of cardiovascular disease, total cancer, and all-cause mortality: a systematic review and dose-response meta-analysis of prospective studies. Nutr Metab Cardiovasc Dis. 2017; 27:504–517. PMID:
28552551.
72. Hillis GS, Woodward M, Rodgers A, Chow CK, Li Q, Zoungas S, Patel A, Webster R, Batty GD, Ninomiya T, Mancia G, Poulter NR, Chalmers J. Resting heart rate and the risk of death and cardiovascular complications in patients with type 2 diabetes mellitus. Diabetologia. 2012; 55:1283–1290. PMID:
22286552.

73. Freeman R, Wieling W, Axelrod FB, Benditt DG, Benarroch E, Biaggioni I, Cheshire WP, Chelimsky T, Cortelli P, Gibbons CH, Goldstein DS, Hainsworth R, Hilz MJ, Jacob G, Kaufmann H, Jordan J, Lipsitz LA, Levine BD, Low PA, Mathias C, Raj SR, Robertson D, Sandroni P, Schatz I, Schondorff R, Stewart JM, van Dijk JG. Consensus statement on the definition of orthostatic hypotension, neutrally mediated syncope and the postural tachycardia syndrome. Clin Auton Res. 2011; 21:69–72. PMID:
21431947.
74. Shibao C, Lipsitz LA, Biaggioni I. ASH position paper: evaluation and treatment of orthostatic hypotension. J Clin Hypertens (Greenwich). 2013; 15:147–153. PMID:
23458585.

75. Low PA, Tomalia VA. Orthostatic hypotension: mechanisms, causes, management. J Clin Neurol. 2015; 11:220–226. PMID:
26174784.

76. Ricci F, Fedorowski A, Radico F, Romanello M, Tatasciore A, Di Nicola M, Zimarino M, De Caterina R. Cardiovascular morbidity and mortality related to orthostatic hypotension: a meta-analysis of prospective observational studies. Eur Heart J. 2015; 36:1609–1617. PMID:
25852216.

77. Spallone V. Blood pressure variability and autonomic dysfunction. Curr Diab Rep. 2018; 18:137. PMID:
30361834.

78. Fleg JL, Evans GW, Margolis KL, Barzilay J, Basile JN, Bigger JT, Cutler JA, Grimm R, Pedley C, Peterson K, Pop-Busui R, Sperl-Hillen J, Cushman WC. Orthostatic hypotension in the ACCORD (Action to Control Cardiovascular Risk in Diabetes) blood pressure trial: prevalence, incidence, and prognostic significance. Hypertension. 2016; 68:888–895. PMID:
27504006.
79. Cuspidi C, Sala C, Tadic M, Gherbesi E, De Giorgi A, Grassi G, Mancia G. Clinical and prognostic significance of a reverse dipping pattern on ambulatory monitoring: an updated review. J Clin Hypertens (Greenwich). 2017; 19:713–721. PMID:
28692165.

80. Fagard RH, Celis H, Thijs L, Staessen JA, Clement DL, De Buyzere ML, De Bacquer DA. Daytime and nighttime blood pressure as predictors of death and cause-specific cardiovascular events in hypertension. Hypertension. 2008; 51:55–61. PMID:
18039980.

81. Hansen TW, Li Y, Boggia J, Thijs L, Richart T, Staessen JA. Predictive role of the nighttime blood pressure. Hypertension. 2011; 57:3–10. PMID:
21079049.

82. Salles GF, Reboldi G, Fagard RH, Cardoso CR, Pierdomenico SD, Verdecchia P, Eguchi K, Kario K, Hoshide S, Polonia J, de la Sierra A, Hermida RC, Dolan E, O'Brien E, Roush GC. ABC-H Investigators. Prognostic effect of the nocturnal blood pressure fall in hypertensive patients: the ambulatory blood pressure collaboration in patients with hypertension (ABC-H) meta-analysis. Hypertension. 2016; 67:693–700. PMID:
26902495.
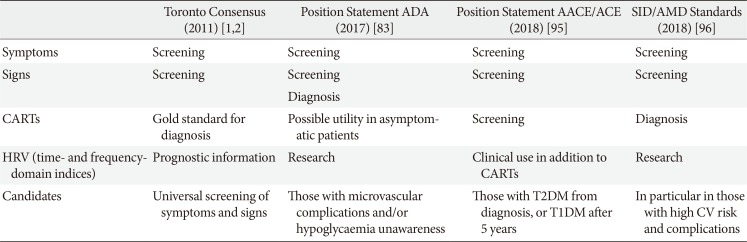
83. Pop-Busui R, Boulton AJ, Feldman EL, Bril V, Freeman R, Malik RA, Sosenko JM, Ziegler D. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care. 2017; 40:136–154. PMID:
27999003.

84. Sletten DM, Suarez GA, Low PA, Mandrekar J, Singer W. COMPASS 31: a refined and abbreviated Composite Autonomic Symptom Score. Mayo Clin Proc. 2012; 87:1196–1201. PMID:
23218087.

85. Greco C, Di Gennaro F, D'Amato C, Morganti R, Corradini D, Sun A, Longo S, Lauro D, Pierangeli G, Cortelli P, Spallone V. Validation of the Composite Autonomic Symptom Score 31 (COMPASS 31) for the assessment of symptoms of autonomic neuropathy in people with diabetes. Diabet Med. 2017; 34:834–838. PMID:
27990686.

86. Kim SH, Lee KA, Jin HY, Baek HS, Park TS. Relationship between the Korean version survey of the autonomic symptoms score and cardiac autonomic neuropathy parameters in patients with diabetic peripheral neuropathy. Diabetes Metab J. 2014; 38:349–355. PMID:
25349822.

87. Spallone V, Morganti R, Fedele T, D'Amato C, Maiello MR. Reappraisal of the diagnostic role of orthostatic hypotension in diabetes. Clin Auton Res. 2009; 19:58–64. PMID:
19199088.

88. Whitsel EA, Boyko EJ, Siscovick DS. Reassessing the role of QTc in the diagnosis of autonomic failure among patients with diabetes: a meta-analysis. Diabetes Care. 2000; 23:241–247. PMID:
10868838.

89. Spallone V, Maiello MR, Morganti R, Mandica S, Frajese G. Usefulness of ambulatory blood pressure monitoring in predicting the presence of autonomic neuropathy in type I diabetic patients. J Hum Hypertens. 2007; 21:381–386. PMID:
17301823.

90. Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, Kahan T, Mahfoud F, Redon J, Ruilope L, Zanchetti A, Kerins M, Kjeldsen SE, Kreutz R, Laurent S, Lip GYH, McManus R, Narkiewicz K, Ruschitzka F, Schmieder RE, Shlyakhto E, Tsioufis C, Aboyans V, Desormais I. ESC Scientific Document Group. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018; 39:3021–3104. PMID:
30165516.

91. Shaw BH, Garland EM, Black BK, Paranjape SY, Shibao CA, Okamoto LE, Gamboa A, Diedrich A, Plummer WD, Dupont WD, Biaggioni I, Robertson D, Raj SR. Optimal diagnostic thresholds for diagnosis of orthostatic hypotension with a ‘sit-to-stand test’. J Hypertens. 2017; 35:1019–1025. PMID:
28129252.

92. Casiglia E, Jordan J. Orthostatic hypotension: new views for an old problem. J Hypertens. 2017; 35:947–949. PMID:
28353545.
93. England JD, Gronseth GS, Franklin G, Carter GT, Kinsella LJ, Cohen JA, Asbury AK, Szigeti K, Lupski JR, Latov N, Lewis RA, Low PA, Fisher MA, Herrmann DN, Howard JF Jr, Lauria G, Miller RG, Polydefkis M, Sumner AJ. American Academy of Neurology. Practice parameter: evaluation of distal symmetric polyneuropathy: role of autonomic testing, nerve biopsy, and skin biopsy (an evidence-based review). Report of the American Academy of Neurology, American Association of Neuromuscular and Electrodiagnostic Medicine, and American Academy of Physical Medicine and Rehabilitation. Neurology. 2009; 72:177–184. PMID:
19056667.
94. Spallone V, Bellavere F, Scionti L, Maule S, Quadri R, Bax G, Melga P, Viviani GL, Esposito K, Morganti R, Cortelli P. Diabetic Neuropathy Study Group of the Italian Society of Diabetology. Recommendations for the use of cardiovascular tests in diagnosing diabetic autonomic neuropathy. Nutr Metab Cardiovasc Dis. 2011; 21:69–78. PMID:
21247746.

95. Vinik AI, Camacho PM, Davidson JA, Handelsman Y, Lando HM, Leddy AL, Reddy SK, Cook R, Spallone V, Tesfaye S, Ziegler D. Task Force to Develop an AACE Position Statement on Autonomic Testing. American Association of Clinical Endocrinologists and American College of Endocrinology position statement on testing for autonomic and somatic nerve dysfunction. Endocr Pract. 2017; 23:1472–1478. PMID:
29320641.

97. Ge X, Pan SM, Zeng F, Tang ZH, Wang YW. A simple Chinese risk score model for screening cardiovascular autonomic neuropathy. PLoS One. 2014; 9:e89623. PMID:
24621478.

98. Charles M, Fleischer J, Witte DR, Ejskjaer N, Borch-Johnsen K, Lauritzen T, Sandbaek A. Impact of early detection and treatment of diabetes on the 6-year prevalence of cardiac autonomic neuropathy in people with screen-detected diabetes: ADDITION-Denmark, a cluster-randomised study. Diabetologia. 2013; 56:101–108. PMID:
23064291.

99. May O, Arildsen H. Assessing cardiovascular autonomic neuropathy in diabetes mellitus: how many tests to use? J Diabetes Complications. 2000; 14:7–12. PMID:
10925060.
100. Pafili K, Trypsianis G, Papazoglou D, Maltezos E, Papanas N. Simplified diagnosis of cardiovascular autonomic neuropathy in type 2 diabetes using Ewing's battery. Rev Diabet Stud. 2015; 12:213–219. PMID:
26676669.

101. Bellavere F, Ragazzi E, Chilelli NC, Lapolla A, Bax G. Autonomic testing: which value for each cardiovascular test? An observational study. Acta Diabetol. 2019; 56:39–43. PMID:
30159748.

102. Tang ZH, Wang L, Zeng F, Li Z, Yu X, Zhang K, Zhou L. Bayesian estimation of cardiovascular autonomic neuropathy diagnostic test based on short-term heart rate variability without a gold standard. BMJ Open. 2014; 4:e005096.

103. Carnethon MR, Prineas RJ, Temprosa M, Zhang ZM, Uwaifo G, Molitch ME. Diabetes Prevention Program Research Group. The association among autonomic nervous system function, incident diabetes, and intervention arm in the Diabetes Prevention Program. Diabetes Care. 2006; 29:914–919. PMID:
16567837.

104. Spallone V, Morganti R, Siampli M, Fedele T, D'Amato C, Cacciotti L, Maiello MR. Neuropad as a diagnostic tool for diabetic autonomic and sensorimotor neuropathy. Diabet Med. 2009; 26:686–692. PMID:
19573117.

105. Papanas N, Boulton AJ, Malik RA, Manes C, Schnell O, Spallone V, Tentolouris N, Tesfaye S, Valensi P, Ziegler D, Kempler P. A simple new non-invasive sweat indicator test for the diagnosis of diabetic neuropathy. Diabet Med. 2013; 30:525–534. PMID:
22924579.

106. Selvarajah D, Cash T, Davies J, Sankar A, Rao G, Grieg M, Pallai S, Gandhi R, Wilkinson ID, Tesfaye S. SUDOSCAN: a simple, rapid, and objective method with potential for screening for diabetic peripheral neuropathy. PLoS One. 2015; 10:e0138224. PMID:
26457582.

107. Ang L, Jaiswal M, Callaghan B, Raffel D, B Brown M, Pop-Busui R. Sudomotor dysfunction as a measure of small fiber neuropathy in type 1 diabetes. Auton Neurosci. 2017; 205:87–92. PMID:
28325598.

108. Casellini CM, Parson HK, Hodges K, Edwards JF, Lieb DC, Wohlgemuth SD, Vinik AI. Bariatric surgery restores cardiac and sudomotor autonomic c-fiber dysfunction towards normal in obese subjects with type 2 diabetes. PLoS One. 2016; 11:e0154211. PMID:
27137224.

109. Novak P. Electrochemical skin conductance: a systematic review. Clin Auton Res. 2019; 29:17–29. PMID:
28951985.

110. Rajan S, Campagnolo M, Callaghan B, Gibbons CH. Sudomotor function testing by electrochemical skin conductance: does it really measure sudomotor function? Clin Auton Res. 2019; 29:31–39. PMID:
29956008.

111. Tavakoli M, Begum P, McLaughlin J, Malik RA. Corneal confocal microscopy for the diagnosis of diabetic autonomic neuropathy. Muscle Nerve. 2015; 52:363–370. PMID:
25556884.

112. Pennathur S, Jaiswal M, Vivekanandan-Giri A, White EA, Ang L, Raffel DM, Rubenfire M, Pop-Busui R. Structured lifestyle intervention in patients with the metabolic syndrome mitigates oxidative stress but fails to improve measures of cardiovascular autonomic neuropathy. J Diabetes Complications. 2017; 31:1437–1443. PMID:
28709739.

113. Alam I, Lewis MJ, Lewis KE, Stephens JW, Baxter JN. Influence of bariatric surgery on indices of cardiac autonomic control. Auton Neurosci. 2009; 151:168–173. PMID:
19720569.

114. Sjoberg N, Brinkworth GD, Wycherley TP, Noakes M, Saint DA. Moderate weight loss improves heart rate variability in overweight and obese adults with type 2 diabetes. J Appl Physiol (1985). 2011; 110:1060–1064. PMID:
21212252.

115. Maser RE, Lenhard MJ, Peters MB, Irgau I, Wynn GM. Effects of surgically induced weight loss by Roux-en-Y gastric bypass on cardiovascular autonomic nerve function. Surg Obes Relat Dis. 2013; 9:221–226. PMID:
22222304.

116. Kokkinos A, Alexiadou K, Liaskos C, Argyrakopoulou G, Balla I, Tentolouris N, Moyssakis I, Katsilambros N, Vafiadis I, Alexandrou A, Diamantis T. Improvement in cardiovascular indices after Roux-en-Y gastric bypass or sleeve gastrectomy for morbid obesity. Obes Surg. 2013; 23:31–38. PMID:
22923313.

117. Lips MA, de Groot GH, De Kam M, Berends FJ, Wiezer R, Van Wagensveld BA, Swank DJ, Luijten A, Pijl H, Burggraaf J. Autonomic nervous system activity in diabetic and healthy obese female subjects and the effect of distinct weight loss strategies. Eur J Endocrinol. 2013; 169:383–390. PMID:
23847327.

118. Ziegler D, Strom A, Nowotny B, Zahiragic L, Nowotny PJ, Carstensen-Kirberg M, Herder C, Roden M. Effect of low-energy diets differing in fiber, red meat, and coffee intake on cardiac autonomic function in obese individuals with type 2 diabetes. Diabetes Care. 2015; 38:1750–1757. PMID:
26070589.

119. Hansen AL, Dahl L, Olson G, Thornton D, Graff IE, Froyland L, Thayer JF, Pallesen S. Fish consumption, sleep, daily functioning, and heart rate variability. J Clin Sleep Med. 2014; 10:567–575. PMID:
24812543.

120. Sauder KA, McCrea CE, Ulbrecht JS, Kris-Etherton PM, West SG. Pistachio nut consumption modifies systemic hemodynamics, increases heart rate variability, and reduces ambulatory blood pressure in well-controlled type 2 diabetes: a randomized trial. J Am Heart Assoc. 2014; 3:e000873. PMID:
24980134.

121. Loimaala A, Huikuri HV, Koobi T, Rinne M, Nenonen A, Vuori I. Exercise training improves baroreflex sensitivity in type 2 diabetes. Diabetes. 2003; 52:1837–1842. PMID:
12829654.

122. Voulgari C, Pagoni S, Vinik A, Poirier P. Exercise improves cardiac autonomic function in obesity and diabetes. Metabolism. 2013; 62:609–621. PMID:
23084034.

123. Villafaina S, Collado-Mateo D, Fuentes JP, Merellano-Navarro E, Gusi N. Physical exercise improves heart rate variability in patients with type 2 diabetes: a systematic review. Curr Diab Rep. 2017; 17:110. PMID:
28942507.

124. Rohling M, Strom A, Bonhof GJ, Roden M, Ziegler D. Cardiorespiratory fitness and cardiac autonomic function in diabetes. Curr Diab Rep. 2017; 17:125. PMID:
29063207.

125. Bhati P, Shenoy S, Hussain ME. Exercise training and cardiac autonomic function in type 2 diabetes mellitus: a systematic review. Diabetes Metab Syndr. 2018; 12:69–78. PMID:
28888482.

126. Bellavere F, Cacciatori V, Bacchi E, Gemma ML, Raimondo D, Negri C, Thomaseth K, Muggeo M, Bonora E, Moghetti P. Effects of aerobic or resistance exercise training on cardiovascular autonomic function of subjects with type 2 diabetes: a pilot study. Nutr Metab Cardiovasc Dis. 2018; 28:226–233. PMID:
29402509.

127. Rosengard-Barlund M, Bernardi L, Fagerudd J, Mantysaari M, Af Bjorkesten CG, Lindholm H, Forsblom C, Waden J, Groop PH. FinnDiane Study Group. Early autonomic dysfunction in type 1 diabetes: a reversible disorder? Diabetologia. 2009; 52:1164–1172. PMID:
19340407.

128. Bernardi L, Bianchi L. Integrated cardio-respiratory control: insight in diabetes. Curr Diab Rep. 2016; 16:107. PMID:
27664040.

129. Gaede P, Oellgaard J, Carstensen B, Rossing P, Lund-Andersen H, Parving HH, Pedersen O. Years of life gained by multifactorial intervention in patients with type 2 diabetes mellitus and microalbuminuria: 21 years follow-up on the Steno-2 randomised trial. Diabetologia. 2016; 59:2298–2307. PMID:
27531506.
130. Manzella D, Grella R, Esposito K, Giugliano D, Barbagallo M, Paolisso G. Blood pressure and cardiac autonomic nervous system in obese type 2 diabetic patients: effect of metformin administration. Am J Hypertens. 2004; 17:223–227. PMID:
15001195.

131. DiBona GF, Esler M. Translational medicine: the antihypertensive effect of renal denervation. Am J Physiol Regul Integr Comp Physiol. 2010; 298:R245–R253. PMID:
19955493.

132. Ghezzi C, Loo DDF, Wright EM. Physiology of renal glucose handling via SGLT1, SGLT2 and GLUT2. Diabetologia. 2018; 61:2087–2097. PMID:
30132032.

133. Matthews VB, Elliot RH, Rudnicka C, Hricova J, Herat L, Schlaich MP. Role of the sympathetic nervous system in regulation of the sodium glucose cotransporter 2. J Hypertens. 2017; 35:2059–2068. PMID:
28598954.

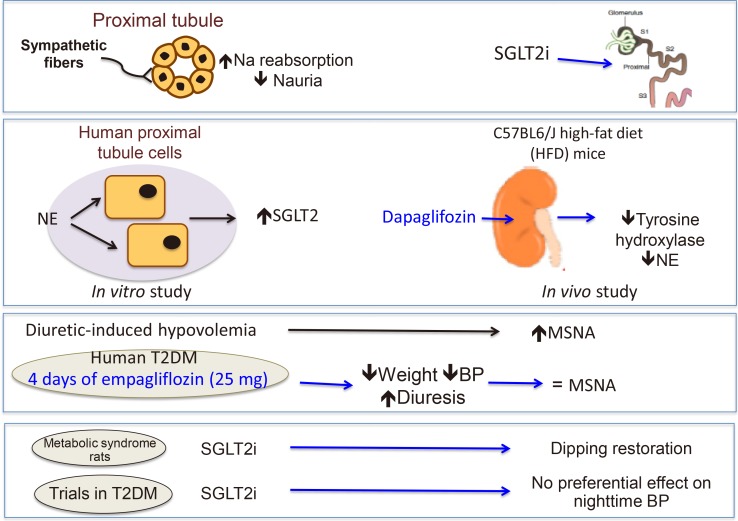
134. Jordan J, Tank J, Heusser K, Heise T, Wanner C, Heer M, Macha S, Mattheus M, Lund SS, Woerle HJ, Broedl UC. The effect of empagliflozin on muscle sympathetic nerve activity in patients with type II diabetes mellitus. J Am Soc Hypertens. 2017; 11:604–612. PMID:
28757109.

135. Rahman A, Fujisawa Y, Nakano D, Hitomi H, Nishiyama A. Effect of a selective SGLT2 inhibitor, luseogliflozin, on circadian rhythm of sympathetic nervous function and locomotor activities in metabolic syndrome rats. Clin Exp Pharmacol Physiol. 2017; 44:522–525. PMID:
28063156.

136. Chilton R, Tikkanen I, Hehnke U, Woerle HJ, Johansen OE. Impact of empagliflozin on blood pressure in dipper and nondipper patients with type 2 diabetes mellitus and hypertension. Diabetes Obes Metab. 2017; 19:1620–1624. PMID:
28387058.

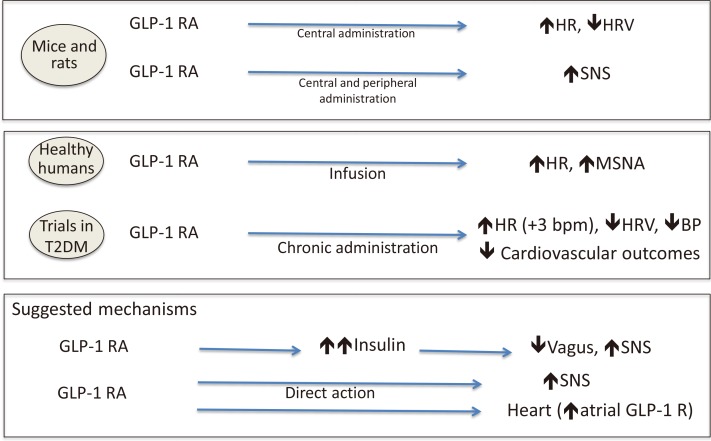
137. Yamamoto H, Lee CE, Marcus JN, Williams TD, Overton JM, Lopez ME, Hollenberg AN, Baggio L, Saper CB, Drucker DJ, Elmquist JK. Glucagon-like peptide-1 receptor stimulation increases blood pressure and heart rate and activates autonomic regulatory neurons. J Clin Invest. 2002; 110:43–52. PMID:
12093887.

138. Valensi P, Chiheb S, Fysekidis M. Insulin- and glucagon-like peptide-1-induced changes in heart rate and vagosympathetic activity: why they matter. Diabetologia. 2013; 56:1196–1200. PMID:
23584434.

139. Bharucha AE, Charkoudian N, Andrews CN, Camilleri M, Sletten D, Zinsmeister AR, Low PA. Effects of glucagon-like peptide-1, yohimbine, and nitrergic modulation on sympathetic and parasympathetic activity in humans. Am J Physiol Regul Integr Comp Physiol. 2008; 295:R874–R880. PMID:
18596108.

140. Sun F, Wu S, Guo S, Yu K, Yang Z, Li L, Zhang Y, Quan X, Ji L, Zhan S. Impact of GLP-1 receptor agonists on blood pressure, heart rate and hypertension among patients with type 2 diabetes: a systematic review and network meta-analysis. Diabetes Res Clin Pract. 2015; 110:26–37. PMID:
26358202.

141. Kumarathurai P, Anholm C, Larsen BS, Olsen RH, Madsbad S, Kristiansen O, Nielsen OW, Haugaard SB, Sajadieh A. Effects of liraglutide on heart rate and heart rate variability: a randomized, double-blind, placebo-controlled crossover study. Diabetes Care. 2017; 40:117–124. PMID:
27797930.

142. Nakatani Y, Kawabe A, Matsumura M, Aso Y, Yasu T, Banba N, Nakamoto T. Effects of GLP-1 receptor agonists on heart rate and the autonomic nervous system using holter electrocardiography and power spectrum analysis of heart rate variability. Diabetes Care. 2016; 39:e22–e23. PMID:
26681718.

143. Cacciatori V, Zoppini G, Bellavere F, Rigolon R, Thomaseth K, Pichiri I, Trombetta M, Dauriz M, De Santi F, Targher G, Santi L, Bonora E. Long-acting GLP-1 receptor agonist exenatide influence on the autonomic cardiac sympatho-vagal balance. J Endocr Soc. 2017; 2:53–62. PMID:
29379894.

144. Smits MM, Muskiet MH, Tonneijck L, Hoekstra T, Kramer MH, Diamant M, van Raalte DH. Exenatide acutely increases heart rate in parallel with augmented sympathetic nervous system activation in healthy overweight males. Br J Clin Pharmacol. 2016; 81:613–620. PMID:
26609792.

145. Baggio LL, Ussher JR, McLean BA, Cao X, Kabir MG, Mulvihill EE, Mighiu AS, Zhang H, Ludwig A, Seeley RJ, Heximer SP, Drucker DJ. The autonomic nervous system and cardiac GLP-1 receptors control heart rate in mice. Mol Metab. 2017; 6:1339–1349. PMID:
29107282.

146. Akbari M, Ostadmohammadi V, Lankarani KB, Tabrizi R, Kolahdooz F, Khatibi SR, Asemi Z. The effects of alpha-lipoic acid supplementation on glucose control and lipid profiles among patients with metabolic diseases: a systematic review and meta-analysis of randomized controlled trials. Metabolism. 2018; 87:56–69. PMID:
29990473.

147. Saboori S, Falahi E, Eslampour E, Zeinali Khosroshahi M, Yousefi Rad E. Effects of alpha-lipoic acid supplementation on C-reactive protein level: a systematic review and meta-analysis of randomized controlled clinical trials. Nutr Metab Cardiovasc Dis. 2018; 28:779–786.

148. Golbidi S, Badran M, Laher I. Diabetes and alpha lipoic acid. Front Pharmacol. 2011; 2:69. PMID:
22125537.

149. Ziegler D, Schatz H, Conrad F, Gries FA, Ulrich H, Reichel G. Effects of treatment with the antioxidant alpha-lipoic acid on cardiac autonomic neuropathy in NIDDM patients. A 4-month randomized controlled multicenter trial (DEKAN Study). Deutsche Kardiale Autonome Neuropathie. Diabetes Care. 1997; 20:369–373. PMID:
9051389.
150. Tankova T, Koev D, Dakovska L. Alpha-lipoic acid in the treatment of autonomic diabetic neuropathy (controlled, randomized, open-label study). Rom J Intern Med. 2004; 42:457–464. PMID:
15529636.
151. Lee SJ, Jeong SJ, Lee YC, Lee YH, Lee JE, Kim CH, Min KW, Cha BY. Effects of high-dose α-lipoic acid on heart rate variability of type 2 diabetes mellitus patients with cardiac autonomic neuropathy in Korea. Diabetes Metab J. 2017; 41:275–283. PMID:
28868825.

152. Pop-Busui R, Stevens MJ, Raffel DM, White EA, Mehta M, Plunkett CD, Brown MB, Feldman EL. Effects of triple antioxidant therapy on measures of cardiovascular autonomic neuropathy and on myocardial blood flow in type 1 diabetes: a randomised controlled trial. Diabetologia. 2013; 56:1835–1844. PMID:
23740194.

153. Ziegler D, Low PA, Freeman R, Tritschler H, Vinik AI. Predictors of improvement and progression of diabetic polyneuropathy following treatment with α-lipoic acid for 4 years in the NATHAN 1 trial. J Diabetes Complications. 2016; 30:350–356. PMID:
26651260.
154. Hu X, Li S, Yang G, Liu H, Boden G, Li L. Efficacy and safety of aldose reductase inhibitor for the treatment of diabetic cardiovascular autonomic neuropathy: systematic review and meta-analysis. PLoS One. 2014; 9:e87096. PMID:
24533052.

155. Johansson BL, Borg K, Fernqvist-Forbes E, Kernell A, Odergren T, Wahren J. Beneficial effects of C-peptide on incipient nephropathy and neuropathy in patients with type 1 diabetes mellitus. Diabet Med. 2000; 17:181–189. PMID:
10784221.

156. Manzella D, Barbieri M, Ragno E, Paolisso G. Chronic administration of pharmacologic doses of vitamin E improves the cardiac autonomic nervous system in patients with type 2 diabetes. Am J Clin Nutr. 2001; 73:1052–1057. PMID:
11382659.

157. Kontopoulos AG, Athyros VG, Didangelos TP, Papageorgiou AA, Avramidis MJ, Mayroudi MC, Karamitsos DT. Effect of chronic quinapril administration on heart rate variability in patients with diabetic autonomic neuropathy. Diabetes Care. 1997; 20:355–361. PMID:
9051387.

158. Malik RA, Williamson S, Abbott C, Carrington AL, Iqbal J, Schady W, Boulton AJ. Effect of angiotensin-converting-enzyme (ACE) inhibitor trandolapril on human diabetic neuropathy: randomised double-blind controlled trial. Lancet. 1998; 352:1978–1981. PMID:
9872248.

159. Athyros VG, Didangelos TP, Karamitsos DT, Papageorgiou AA, Boudoulas H, Kontopoulos AG. Long-term effect of converting enzyme inhibition on circadian sympathetic and parasympathetic modulation in patients with diabetic autonomic neuropathy. Acta Cardiol. 1998; 53:201–209. PMID:
9842405.
160. Kubba S, Agarwal SK, Prakash A, Puri V, Babbar R, Anuradha S. Effect of losartan on albuminuria, peripheral and autonomic neuropathy in normotensive microalbuminuric type 2 diabetics. Neurol India. 2003; 51:355–358. PMID:
14652437.
161. Maser RE, Lenhard MJ. Effect of treatment with losartan on cardiovascular autonomic and large sensory nerve fiber function in individuals with diabetes mellitus: a 1-year randomized, controlled trial. J Diabetes Complications. 2003; 17:286–291. PMID:
12954158.
162. Didangelos TP, Arsos GA, Karamitsos DT, Athyros VG, Georga SD, Karatzas ND. Effect of quinapril or losartan alone and in combination on left ventricular systolic and diastolic functions in asymptomatic patients with diabetic autonomic neuropathy. J Diabetes Complications. 2006; 20:1–7. PMID:
16389160.

163. Ebbehoj E, Poulsen PL, Hansen KW, Knudsen ST, Molgaard H, Mogensen CE. Effects on heart rate variability of metoprolol supplementary to ongoing ACE-inhibitor treatment in Type I diabetic patients with abnormal albuminuria. Diabetologia. 2002; 45:965–975. PMID:
12136395.

164. Gibbons CH, Schmidt P, Biaggioni I, Frazier-Mills C, Freeman R, Isaacson S, Karabin B, Kuritzky L, Lew M, Low P, Mehdirad A, Raj SR, Vernino S, Kaufmann H. The recommendations of a consensus panel for the screening, diagnosis, and treatment of neurogenic orthostatic hypotension and associated supine hypertension. J Neurol. 2017; 264:1567–1582. PMID:
28050656.

165. Fanciulli A, Jordan J, Biaggioni I, Calandra-Buonaura G, Cheshire WP, Cortelli P, Eschlboeck S, Grassi G, Hilz MJ, Kaufmann H, Lahrmann H, Mancia G, Mayer G, Norcliffe-Kaufmann L, Pavy-Le Traon A, Raj SR, Robertson D, Rocha I, Struhal W, Thijs R, Tsioufis KP, van Dijk JG, Wenning GK. Consensus statement on the definition of neurogenic supine hypertension in cardiovascular autonomic failure by the American Autonomic Society (AAS) and the European Federation of Autonomic Societies (EFAS): Endorsed by the European Academy of Neurology (EAN) and the European Society of Hypertension (ESH). Clin Auton Res. 2018; 28:355–362.
166. Zhao P, Xu P, Wan C, Wang Z. Evening versus morning dosing regimen drug therapy for hypertension. Cochrane Database Syst Rev. 2011; (10):CD004184. PMID:
21975743.

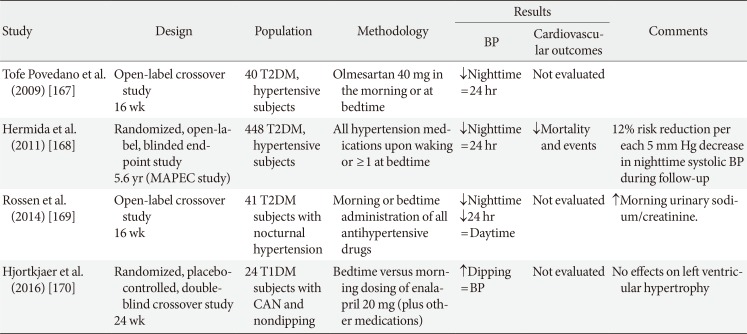
167. Tofe Povedano S, Garcia De La Villa B. 24-Hour and nighttime blood pressures in type 2 diabetic hypertensive patients following morning or evening administration of olmesartan. J Clin Hypertens (Greenwich). 2009; 11:426–431. PMID:
19695030.
168. Hermida RC, Ayala DE, Mojon A, Fernandez JR. Influence of time of day of blood pressure-lowering treatment on cardiovascular risk in hypertensive patients with type 2 diabetes. Diabetes Care. 2011; 34:1270–1276. PMID:
21617110.

169. Rossen NB, Knudsen ST, Fleischer J, Hvas AM, Ebbehoj E, Poulsen PL, Hansen KW. Targeting nocturnal hypertension in type 2 diabetes mellitus. Hypertension. 2014; 64:1080–1087. PMID:
25259747.

170. Hjortkjaer HO, Jensen T, Kofoed KF, Mogensen UM, Sigvardsen PE, Kober L, Hilsted KL, Corinth H, Theilade S, Hilsted J. Nocturnal antihypertensive treatment in patients with type 1 diabetes with autonomic neuropathy and non-dipping: a randomised, placebo-controlled, double-blind cross-over trial. BMJ Open. 2016; 6:e012307.

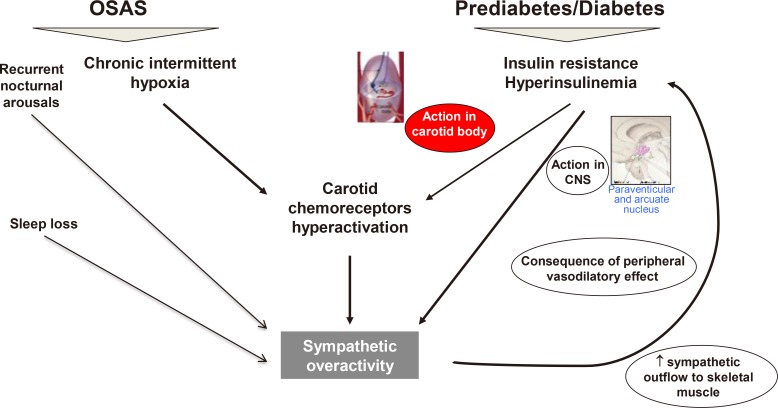
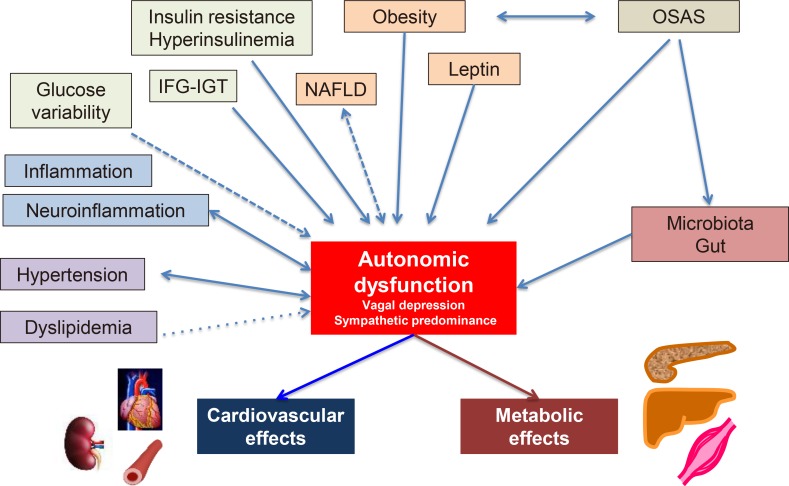
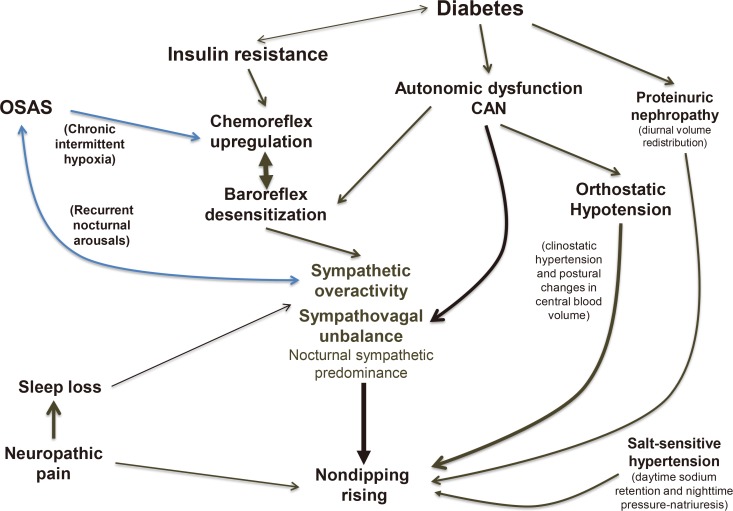
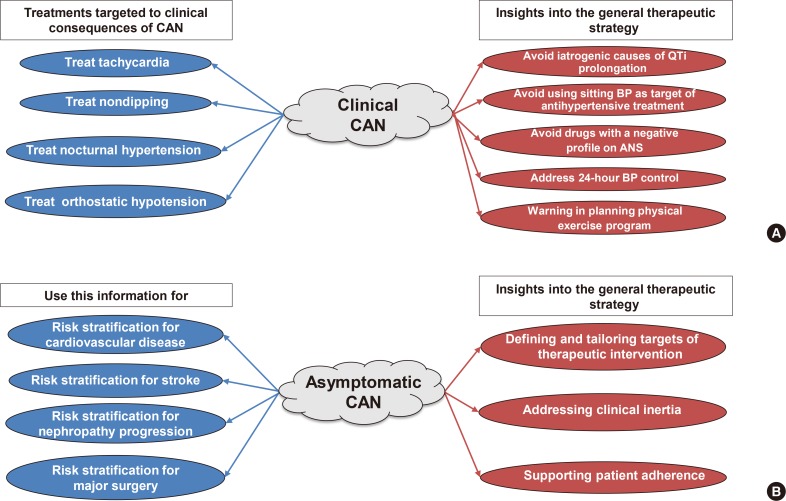




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download