1. Moran AE, Forouzanfar MH, Roth GA, Mensah GA, Ezzati M, Murray CJ, Naghavi M. Temporal trends in ischemic heart disease mortality in 21 world regions, 1980 to 2010: the Global Burden of Disease 2010 study. Circulation. 2014; 129:1483–1492. PMID:
24573352.
2. Roth GA, Forouzanfar MH, Moran AE, Barber R, Nguyen G, Feigin VL, Naghavi M, Mensah GA, Murray CJ. Demographic and epidemiologic drivers of global cardiovascular mortality. N Engl J Med. 2015; 372:1333–1341. PMID:
25830423.

3. Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature. 2006; 444:875–880. PMID:
17167476.

4. Rexrode KM, Carey VJ, Hennekens CH, Walters EE, Colditz GA, Stampfer MJ, Willett WC, Manson JE. Abdominal adiposity and coronary heart disease in women. JAMA. 1998; 280:1843–1848. PMID:
9846779.

5. Yusuf S, Hawken S, Ounpuu S, Bautista L, Franzosi MG, Commerford P, Lang CC, Rumboldt Z, Onen CL, Lisheng L, Tanomsup S, Wangai P Jr, Razak F, Sharma AM, Anand SS. INTERHEART Study Investigators. Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: a case-control study. Lancet. 2005; 366:1640–1649. PMID:
16271645.

6. de Koning L, Merchant AT, Pogue J, Anand SS. Waist circumference and waist-to-hip ratio as predictors of cardiovascular events: meta-regression analysis of prospective studies. Eur Heart J. 2007; 28:850–856. PMID:
17403720.

7. Yajnik CS, Yudkin JS. The Y-Y paradox. Lancet. 2004; 363:163. PMID:
14726172.

8. Oh HG, Nallamshetty S, Rhee EJ. Increased risk of progression of coronary artery calcification in male subjects with high baseline waist-to-height ratio: the Kangbuk Samsung Health Study. Diabetes Metab J. 2016; 40:54–61. PMID:
26912156.

9. Mathieu P, Pibarot P, Larose E, Poirier P, Marette A, Despres JP. Visceral obesity and the heart. Int J Biochem Cell Biol. 2008; 40:821–836. PMID:
18201922.

10. Xu Z, Qi X, Dahl AK, Xu W. Waist-to-height ratio is the best indicator for undiagnosed type 2 diabetes. Diabet Med. 2013; 30:e201–e207. PMID:
23444984.
11. Browning LM, Hsieh SD, Ashwell M. A systematic review of waist-to-height ratio as a screening tool for the prediction of cardiovascular disease and diabetes: 0.5 could be a suitable global boundary value. Nutr Res Rev. 2010; 23:247–269. PMID:
20819243.

12. World Health Organization. Waist circumference and waist-hip ratio: report of a WHO expert consultation. Geneva: World Health Organization;2011.
13. Alberti KG, Zimmet P, Shaw J. Metabolic syndrome: a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med. 2006; 23:469–480. PMID:
16681555.
14. Tan CE, Ma S, Wai D, Chew SK, Tai ES. Can we apply the National Cholesterol Education Program Adult Treatment Panel definition of the metabolic syndrome to Asians. Diabetes Care. 2004; 27:1182–1186. PMID:
15111542.

15. Lear SA, Humphries KH, Kohli S, Chockalingam A, Frohlich JJ, Birmingham CL. Visceral adipose tissue accumulation differs according to ethnic background: results of the Multicultural Community Health Assessment Trial (M-CHAT). Am J Clin Nutr. 2007; 86:353–359. PMID:
17684205.

16. Deurenberg-Yap M, Chew SK, Lin VF, Tan BY, van Staveren WA, Deurenberg P. Relationships between indices of obesity and its co-morbidities in multi-ethnic Singapore. Int J Obes Relat Metab Disord. 2001; 25:1554–1562. PMID:
11673781.

17. Deurenberg-Yap M, Schmidt G, van Staveren WA, Deurenberg P. The paradox of low body mass index and high body fat percentage among Chinese, Malays and Indians in Singapore. Int J Obes Relat Metab Disord. 2000; 24:1011–1017. PMID:
10951540.

18. Yoon YS, Oh SW. Optimal waist circumference cutoff values for the diagnosis of abdominal obesity in korean adults. Endocrinol Metab (Seoul). 2014; 29:418–426. PMID:
25559570.

19. Ekoru K, Murphy GAV, Young EH, Delisle H, Jerome CS, Assah F, Longo-Mbenza B, Nzambi JPD, On'Kin JBK, Buntix F, Muyer MC, Christensen DL, Wesseh CS, Sabir A, Okafor C, Gezawa ID, Puepet F, Enang O, Raimi T, Ohwovoriole E, Oladapo OO, Bovet P, Mollentze W, Unwin N, Gray WK, Walker R, Agoudavi K, Siziya S, Chifamba J, Njelekela M, Fourie CM, Kruger S, Schutte AE, Walsh C, Gareta D, Kamali A, Seeley J, Norris SA, Crowther NJ, Pillay D, Kaleebu P, Motala AA, Sandhu MS. Deriving an optimal threshold of waist circumference for detecting cardiometabolic risk in sub-Saharan Africa. Int J Obes (Lond). 2018; 42:487–494.

20. Song SO, Jung CH, Song YD, Park CY, Kwon HS, Cha BS, Park JY, Lee KU, Ko KS, Lee BW. Background and data configuration process of a nationwide population-based study using the Korean National Health Insurance System. Diabetes Metab J. 2014; 38:395–403. PMID:
25349827.

21. WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004; 363:157–163. PMID:
14726171.
22. Oh SW, Shin SA, Yun YH, Yoo T, Huh BY. Cut-off point of BMI and obesity-related comorbidities and mortality in middle-aged Koreans. Obes Res. 2004; 12:2031–2040. PMID:
15687405.

23. Lee CM, Huxley RR, Wildman RP, Woodward M. Indices of abdominal obesity are better discriminators of cardiovascular risk factors than BMI: a meta-analysis. J Clin Epidemiol. 2008; 61:646–653. PMID:
18359190.

24. Newman AB, Gottdiener JS, Mcburnie MA, Hirsch CH, Kop WJ, Tracy R, Walston JD, Fried LP. Cardiovascular Health Study Research Group. Associations of subclinical cardiovascular disease with frailty. J Gerontol A Biol Sci Med Sci. 2001; 56:M158–M166. PMID:
11253157.

25. Mongraw-Chaffin ML, Peters SAE, Huxley RR, Woodward M. The sex-specific association between BMI and coronary heart disease: a systematic review and meta-analysis of 95 cohorts with 1·2 million participants. Lancet Diabetes Endocrinol. 2015; 3:437–449. PMID:
25960160.

26. Thomsen M, Nordestgaard BG. Myocardial infarction and ischemic heart disease in overweight and obesity with and without metabolic syndrome. JAMA Intern Med. 2014; 174:15–22. PMID:
24217719.

27. Hu G, Tuomilehto J, Silventoinen K, Sarti C, Mannisto S, Jousilahti P. Body mass index, waist circumference, and waist-hip ratio on the risk of total and type-specific stroke. Arch Intern Med. 2007; 167:1420–1427. PMID:
17620537.

28. Yatsuya H, Folsom AR, Yamagishi K, North KE, Brancati FL, Stevens J. Atherosclerosis Risk in Communities Study Investigators. Race- and sex-specific associations of obesity measures with ischemic stroke incidence in the Atherosclerosis Risk in Communities (ARIC) study. Stroke. 2010; 41:417–425. PMID:
20093637.

29. Romero-Corral A, Somers VK, Sierra-Johnson J, Thomas RJ, Collazo-Clavell ML, Korinek J, Allison TG, Batsis JA, Sert-Kuniyoshi FH, Lopez-Jimenez F. Accuracy of body mass index in diagnosing obesity in the adult general population. Int J Obes (Lond). 2008; 32:959–966. PMID:
18283284.

30. Winter Y, Rohrmann S, Linseisen J, Lanczik O, Ringleb PA, Hebebrand J, Back T. Contribution of obesity and abdominal fat mass to risk of stroke and transient ischemic attacks. Stroke. 2008; 39:3145–3151. PMID:
18703800.

31. Ashwell M, Gunn P, Gibson S. Waist-to-height ratio is a better screening tool than waist circumference and BMI for adult cardiometabolic risk factors: systematic review and meta-analysis. Obes Rev. 2012; 13:275–286. PMID:
22106927.

32. Pouliot MC, Despres JP, Lemieux S, Moorjani S, Bouchard C, Tremblay A, Nadeau A, Lupien PJ. Waist circumference and abdominal sagittal diameter: best simple anthropometric indexes of abdominal visceral adipose tissue accumulation and related cardiovascular risk in men and women. Am J Cardiol. 1994; 73:460–468. PMID:
8141087.

33. Rimm EB, Stampfer MJ, Colditz GA, Chute CG, Litin LB, Willett WC. Validity of self-reported waist and hip circumferences in men and women. Epidemiology. 1990; 1:466–473. PMID:
2090285.

34. Nordhamn K, Sodergren E, Olsson E, Karlstrom B, Vessby B, Berglund L. Reliability of anthropometric measurements in overweight and lean subjects: consequences for correlations between anthropometric and other variables. Int J Obes Relat Metab Disord. 2000; 24:652–657. PMID:
10849590.

35. Lee CD, Blair SN, Jackson AS. Cardiorespiratory fitness, body composition, and all-cause and cardiovascular disease mortality in men. Am J Clin Nutr. 1999; 69:373–380. PMID:
10075319.

36. Seimon RV, Wild-Taylor AL, Gibson AA, Harper C, McClintock S, Fernando HA, Hsu MSH, Luz FQD, Keating SE, Johnson NA, Grieve SM, Markovic TP, Caterson ID, Byrne NM, Sainsbury A. Less waste on waist measurements: determination of optimal waist circumference measurement site to predict visceral adipose tissue in postmenopausal women with obesity. Nutrients. 2018; 10:E239. PMID:
29461494.

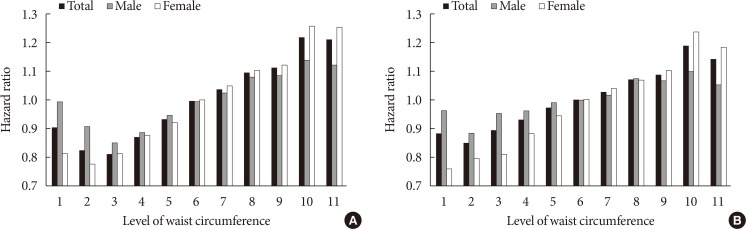
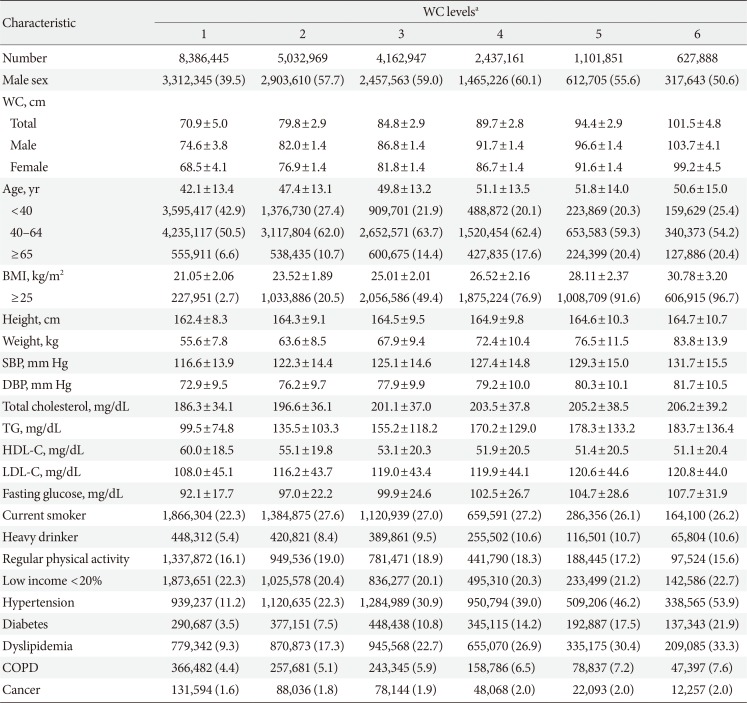
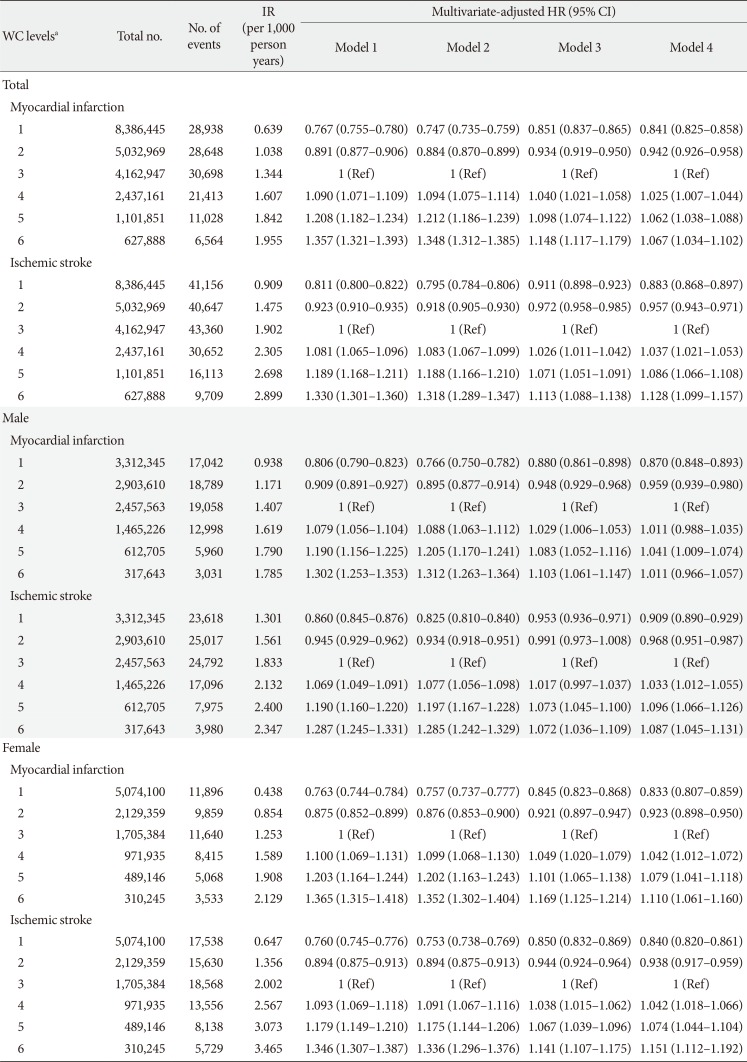
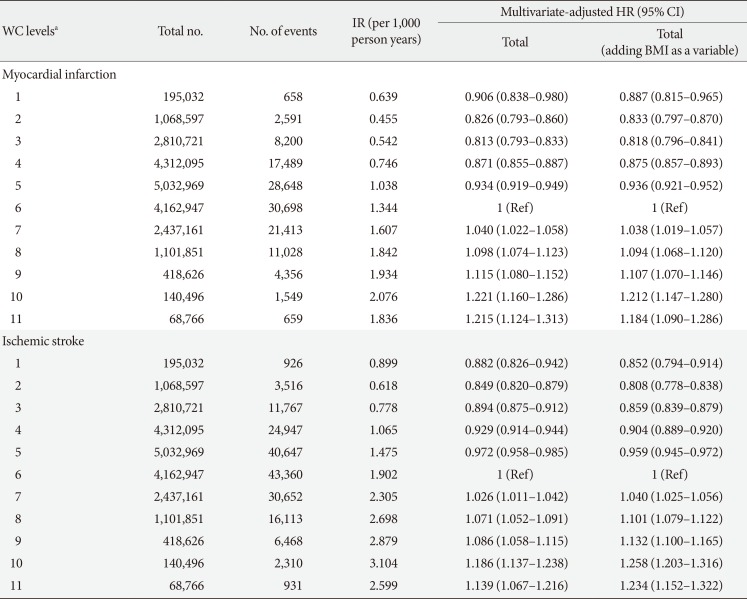




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download