Abstract
Obstructive sleep apnea (OSA) and diabetes has been known to be closely related to each other and both diseases impact highly on the public health. There are many evidence of reports that OSA is associated with diabetes with a bidirectional correlation. A possible causal mechanism of OSA to diabetes is intermittent hypoxemia and diabetes to OSA is microvascular complication. However, OSA and diabetes have a high prevalence rate in public and shares the common overlap characteristic and risk factors such as age, obesity, and metabolic syndrome that make it difficult to establish the exact pathophysiologic mechanism between them. In addition, studies demonstrating that treatment of OSA may help prevent diabetes or improve glycemic control have not shown convincing result but have become a great field of interest research. This review outlines the bidirectional correlation between OSA and diabetes and explore the pathophysiologic mechanisms by approaching their basic etiologies.
Historically, obstructive sleep apnea (OSA) was mistakenly recognized as the Pickwickian syndrome based on observations of obese persons exhibiting symptoms of snoring and sleepiness akin to a character portrayed by Charles Dickens' Pickwick Papers from the early 19th century [1]. It was not until the second half of the 20th century when OSA was established as a standalone entity apart from obesity hypoventilation syndrome [2]. The contemporary definition of OSA is the presence of recurrent respiratory events during sleep characterized by upper airway resistance with continued respiratory effort [3]. Although the main cause of OSA is anatomic narrowing of the upper airway, factors such as pharyngeal muscle responsiveness, central neurohormonal control of breathing, and awakening response to obstruction all play an intricate pathophysiologic role [3].
Adverse effects of OSA can be considered as primary and secondary. The primary effect of upper airway obstruction results in oxygen desaturations, hypoxia, and sleep fragmentation [4]. Secondary effects are more complex and less well described, but result in destabilization of sleep homeostasis and unfavorable changes to the cardiometabolic system [5]. Current research has not clearly distinguished the metabolic outcomes related to OSA induced intermittent hypoxia or recurrent arousals with sleep fragmentation. It may be that these processes share adverse physiologic effects on systemic inflammation, sympathetic surge, and glucose intolerance. In this review, we outline the bidirectional relationship between OSA and metabolic syndrome and discuss the available data on the mechanisms involved.
Based on current 3rd international classification of sleep disorders (ICSD-3) manual, two diagnostic criteria for OSA are proposed. In clinical practice, the diagnostic criteria most commonly fulfilled is based on a combination of OSA compatible symptoms such as habitual snoring, witnessed apneas, awakening with choking or gasping, insomnia, and daytime fatigue or sleepiness along with a sleep study that demonstrates five or more obstructive respiratory events per hour of sleep. Comorbid diseases unrelated to sleep, such as hypertension, coronary artery disease, stroke, heart failure, atrial fibrillation, or diabetes, may be counted in lieu of symptom criteria. Alternatively, a sleep study with 15 or more obstructive respiratory events per hour of sleep meets diagnostic criteria (Table 1).
Specific scoring rules define types of obstructive respiratory events captured on a sleep study that includes apnea, hypopnea, or respiratory effort related arousal (RERA). Apnea refers to a cessation of breathing of at least 10 seconds generally but not always associated with oxygen desaturation. Hypopnea refers to shallow breathing of at least 10 seconds that must be associated with oxygen desaturation or result in an arousal from sleep. RERA refers to any breathing disturbance of at least 10 seconds not meeting above criteria resulting in an arousal from sleep.
The ICSD by American Academy of Sleep Medicine includes two definitions for hypopnea. The recommended hypopnea definition requires a 30% reduction in nasal pressure signal for 10 seconds or longer in association with either an arousal or 3% or greater arterial oxygen desaturation. The alternative definition of hypopnea requires a 30% or greater reduction in the nasal pressure signal associated with a 4% or greater arterial oxygen desaturation [6].
A feature of apnea is the occurrence of intermittent hypoxia during sleep. Hypopnea has features of both intermittent hypoxia and arousal which leads to sleep fragmentation. RERA is mostly associated with unexpected arousals during sleep.
Furthermore, OSA severity is determined by the number of obstructive respiratory events per hour of sleep. The most commonly used parameter in clinical practice and research studies is the apnea-hypopnea index (AHI), a summation of apneas and hypopneas. AHI of <15, 15 to <30, and ≥30 correlates to mild, moderate, and severe OSA respectively. The respiratory disturbance index (RDI) defined as the AHI plus RERA index may also be used as a more inclusive index. However, it is less often used in research and cutoffs points for assessing severity are less clear. RDI is not defined in the ICSD-3 criteria but may have a clinical significance when certain comorbidities like cardiovascular disease, diabetes, hypertension are present [2].
Prevalence of OSA is challenging to define due to changes in consensus definitions of OSA over time, the population sampled, and variance in diagnostic criteria from epidemiologic research. In general population, OSA patients with daytime somnolence occurs in 3% to 7% of adult male and 2% to 5% of adult female [7]. OSA prevalence, however, is as high as 24% in males and 9% in females using only an AHI criterion of ≥5/hour [4]. Although including population of moderate-to-severe OSA (AHI ≥15/hour) patients only, the prevalence was increased as high as 23.4% (95% confidence interval [CI], 20.9 to 26.0) in woman and 49.7% (95% CI, 46.6 to 52.8) in males fulfilling the criterion of 3% oxygen desaturation event [8]. In part, much of the increase is driven by increasing trends in obesity, but also a relaxation of OSA diagnostic criteria due to updated practice guidelines. Previously, authors used hypopnea scoring as 4% oxygen desaturation only or 4% oxygen desaturation/arousal during respiratory event. Recent studies mostly use the criteria of 3% oxygen desaturation or arousal that result in the higher prevalence rate (Table 2) [89101112131415].
The prevalence of OSA in people with type 2 diabetes mellitus (T2DM) is higher than the general population and increases further as OSA severity increases [9]. An OSA prevalence of 86% was observed in 306 people with T2DM with severe obesity body mass index >36.5±5.8 kg/m2 and waist circumference >115.0±13.0 cm [16]. The fact that a high prevalence of OSA has been reported in a type 1 diabetes mellitus (T1DM) adult population raises the possibility of this disorder being associated with not only excess adiposity but also hyperglycemia itself [17]. The prevalence of moderate to severe OSA (AHI ≥15) was 10.3% in T1DM [18] and 46.3% in long-standing T1DM with 29±14 years duration [19].
It is known that T2DM and OSA are closely related and have a major impact on public health [20]. Both diseases are highly prevalent and share common risk factors such as older age and metabolic syndrome which make it difficult to elucidate causal relationships. Many people with OSA are diagnosed with incident T2DM and vice versa [21], yet whether these two conditions are associated causally or reflect a common association with a third factor is not understood.
A possible mechanism linking OSA causally to T2DM is intermittent hypoxia which may provoke β-cell dysfunction and insulin resistance [22]. An alternative hypothesis is an increase in epinephrine, norepinephrine, and cortisol secretion that leads to increased gluconeogenesis and decreased glucose uptake in association with oxyhemoglobin desaturation and hypercarbia [23]. Moreover, it was observed that glucose control represented by higher glycosylated hemoglobin levels was poorer in patients with more severe OSA [24]. Since OSA is more likely in persons who are overweight and obese, and since excess body weight is also a major risk factor for T2DM, whether the T2DM association with OSA is due to OSA mediated changes in glucose metabolism or simply reflects an association in common with excess adiposity is not well understood.
Obesity, OSA, sleep, and metabolic dysregulation are intricately connected. Sleep disturbance may promote behavioral, metabolic, and/or hormonal changes with weight gain. In OSA, the greater energy expenditure due to increased resting metabolic rate may induce a compensatory neuroendocrine adaptation to increase hunger and food intake beyond the requirements of energy balance [25]. This results in positive energy balance and a higher risk of obesity which promotes dyslipidemia, an inflammatory state, and lower insulin sensitivity [26]. OSA diagnosed by polysomnography can be described as a pathophysiologic condition characterized by intermittent hypoxia and arousal. The physiologic changes associated with these two states include oxidative stress due to intermittent hypoxia and sympathetic surge due to arousal. We will describe these phenomena in more detail below.
Glucose homeostasis is impaired by hypoxia that is associated with OSA. Research in 150 middle-aged overweight males with sleep disordered breathing demonstrated a higher odds for worsening glucose tolerance (odds ratio, 1.99; 95% CI, 1.11 to 3.56) in association with a 4% decrease in oxygen saturation [27]. It has also been demonstratedin in vivo kinetic research of glucose metabolism that OSA with severe desaturation impairs insulin sensitivity, glucose effectiveness, and pancreatic β-cell function [28].
Additional clinical research supports and association between hypoxemia and hyperglycemia. Patients with chronic hypoxic respiratory disease showed significantly higher plasma glucose than healthy age and sex matched controls [29]. Exposure to high altitude and related hypoxia has been shown in males to reduce insulin sensitivity [1030]. Even intermittent hypoxia during wakefulness induced a 17% reduction in insulin sensitivity without an increase in insulin secretion [31]. These findings support the plausibility of an association between OSA induced hypoxia and higher risk of T2DM (Fig. 1).
Hypoxic may cause a stress-related increase in hypothalamic-pituitary-adrenal axis activity and higher circulating cortisol concentration. Cortisol interferes with glucose metabolism and increases risk of diabetes [323334]. It has multiple effects on glucose metabolism including inhibition of insulin secretion by modifying β-cell function, increasing hepatic gluconeogenesis, and activation of lipoprotein lipase which modulates nonesterified fatty acids that can decrease insulin sensitivity [3235].
Intermittent hypoxia can also increase sympathetic activity. Hypoxia with hypercarbia in OSA patients elicits baroreflex dysfunction, altered cardiovascular respiratory variation, vasoconstrictor effects of nocturnal endothelin release and vascular endothelial cell dysfunction [536]. Resulting higher sympathetic activity leads to higher blood pressure [23]. Sympathetic excitation can impair tissue sensitivity to insulin levels and result in resistance [37].
Patients with OSA have higher levels of inflammatory cytokines [383940]. An animal model study showed that intermittent hypoxia elevates cytokines and inflammatory mediators such as interleukin 1α (IL-1α), IL-1β, IL-4, IL-6, and IL-13 [41]. Increased levels of inflammatory mediators can worsen systemic or local inflammation. Intermittent hypoxia in OSA induces oxidative stress that triggers the release of inflammatory cytokines and vasoactive substances that may cause endothelial damage. Greater systemic inflammation and higher cytokine concentrations have been linked to greater insulin resistance [42].
Normal sleep architecture cycles between non-rapid eye movement (NREM) (N1, N2, N3 stages) and rapid eye movement (REM) sleep about four times per night. Among NREM sleep, N3 or slow wave sleep is the deepest stage and most important in terms of neurohormonal balance [43]. Arousals may disrupt the sleep cycle by reducing NREM sleep, preferentially stage N3. Subsequent suppression of slow wave sleep results in a 25% decrease in insulin sensitivity without a compensatory increase in insulin secretion [43]. Higher glycosylated hemoglobin levels of T2DM patients correlates with more fragmented sleep [44]. Another study reported that sleep fragmentation was associated with significantly higher fasting glucose and insulin concentrations in persons with diabetes [45]. Sleep fragmentation by arousals also increases sympathetic nervous system activity and this has inhibitory effects on insulin secretion while also decreasing insulin sensitivity [46]. Frequent arousals decrease sleep efficiency and ultimately result in sleep deprivation. Recurrent sleep restriction reduces oral glucose tolerance and insulin sensitivity and increases epinephrine and norepinephrine concentration at nighttime [47]. Elevations of the concentrations of these counterregulatory catecholamines result in gluconeogenesis and elevated glucose concentration [48]. The sleep deprived state also reduces appetite regulating hormones such as leptin which increases hunger and predisposes to excess caloric intake leading to obesity and impaired glucose metabolism [4950].
Both sleep fragmentation and deprivation elevate evening cortisol which may lead to morning insulin resistance [47]. Experimental studies of normal volunteers showed that sleep deprivation is associated with impaired glucocorticoid regulation and abnormal glucose tolerance [5152]. Insufficient sleep is related to reduced insulin sensitivity and higher risk of diabetes and short sleep duration of less than 6 hours increases the odds of both prediabetes and diabetes [53].
The circadian rhythm is an endogenous physiologic process lasting approximately 24 hours regulated by the suprachiasmatic nucleus of the anterior hypothalamus, which serves as the central neural pacemaker of the sleep wake cycle [54]. This rhythm is also influenced by external stimuli such as the diurnal light-dark cycle. Multiple metabolic processes and the timing of hormonal secretion are regulated by this circadian rhythm [55]. Cortisol is low at the beginning of sleep onset, rises during the sleep cycle, and peaks just before the end of the sleep cycle. Growth hormone, prolactin, and parathyroid hormone show increased levels during sleep [56].
Arousals may destabilize the circadian rhythm by disrupting the normal sleep-wake cycle resulting in circadian misalignment. Circadian misalignment cause metabolic alterations associated with higher risk in diabetes [5758]. The disruption of circadian rhythm by alteration of behavioral cycles (sleep/wake, fasting/feeding, and activity schedules) effects glucose and lipid metabolism that may result in the development of obesity, hypertension, hyperlipidemia, and hyperglycemia [5960].
Important neuroendocrine changes are associated with arousals from sleep. Melatonin is secreted by the pineal gland and levels peak at night. This hormone is controlled by the suprachiasmatic nucleus and modifies central circadian clock by light signal via the retinohypothalamic tract [56]. Sleep disruptions by arousals in OSA may modify circadian rhythm and melatonin secretion. Research has revealed lower melatonin levels in patients with OSA during night sleep assessed with the urinary 6-sulfatoxymelatonin/creatine ratio [61]. Melatonin receptor isoforms are detected in the pancreatic β-cells and α-cells, and melatonin has been shown to modulate insulin secretion [62]. More severe OSA was significantly correlated with lower nocturnal melatonin level, and low melatonin level was observed to be associated with poor glycemic control [63]. The fact that lower nocturnal level of melatonin was found in T2DM patients compared with controls suggests the possibility that lower melatonon concentration may increase T2DM risk [64].
Arousals are known to increase sympathetic activity [65]. Patients with OSA have hyperactivity of the sympathetic nervous system with higher epinephrine/norepinephrine and urinary catecholamine levels following arousal [46]. Catecholamines increases hepatic glucose production and reduce insulin sensitivity and insulin-mediated glucose uptake [666768]. Moreover, increased sympathetic activity has lipolytic effects, increasing levels of nonesterified fatty acids, which can worsen insulin sensitivity and glucose tolerance [697071]. Systemic vasoconstriction is another untoward effect of greater sympathetic activity that decreases metabolic rate and glucose uptake in skeletal muscle [7273]. Sympathetic excitation can also interfere with insulin signaling or decrease insulin-mediated glucose uptake by adipocytes [3774].
Diabetes may worsen OSA by altered responsiveness of the carotid body in the hyperglycemic state [75]. Chronic exposure to hyperglycemia attenuates carotid body discharge rate which causes degeneration of carotid body parenchyma and may lead to dampening of hypoxic reactivity [76]. Animal research showed that injection of glucose in the carotid sinus region when isolated from the vasculature reduced the electrical activity of carotid body chemoreceptors and increased their threshold to hypoxia [77]. Decreased sensitivity of the carotid body by hyperglycemia impairs ventilatory responses in mice and may therefore predispose to OSA in humans [78].
Multiple types of diabetic peripheral neuropathy (DPN) exists and may be roughly grouped into focal or diffuse varieties. Peripheral sensorimotor neuropathy usually involves distal extremities and can be characterized by a “stocking-glove” distribution with higher risk of development from longer exposure to or greater severity of hyperglycemia [79]. Symptoms of paresthesia, dysesthesia, and neuralgias are due to damage of small sensory fibers. Involvement of large fibers impairs proprioception and sensation to light touch. Autonomic neuropathy affects the sympathetic and parasympathetic nervous system resulting in, for example, decreased heart rate variability, neurogenic bladder, disorders of sweating, gastroparesis, erectile dysfunction.
The multiple types of diabetic neuropathy may impact the development or severity of OSA which is more often prevalent in diabetic patients with autonomic neuropathy. It is reported that 26% of those who have diabetes with autonomic neuropathy had mild OSA compared to non-autonomic neuropathy diabetic control group [80]. Autonomic neuropathy may have an impact on the chemical control of breathing by affecting central and peripheral chemoreceptors and glossopharyngeal, vagal, proprioceptive nerves [818283]. Upper airway neuropathy might promote neuromuscular dysfunction of the upper airway dilator muscle leading to narrowing or closure of the upper respiratory tract [1984].
Patients with DPN have a common overlap characteristic of obesity and metabolic syndrome. These conditions are related to increased adipose tissue which may lead to OSA by increased airway tissue [22]. One study showed that obese patients with OSA had excess fat accumulation in the tongue base compared to controls [85]. Increased tongue volume and deposition of fat at the base of tongue may contribute to the high incidence of OSA in obese people.
Melatonin treatment has been investigated as a means to correct or limit the metabolic damage associated with OSA. Melatonin is a hormone that is used to treat insomnia or sleep disorders such as REM sleep behavior disorder, delayed sleep phase disorders, jet lag, or sleep problems associated with shift work [86]. In one animal study, melatonin supplementation showed reduced inflammation, insulin resistance, and microvascular damage from oxidative stress [87]. Another study showed that melatonin prevented endothelial dysfunction through alterations of nitric oxide and antioxidant enzymes concentrations leading to stabilization of vascular inflammation [88]. However there is an evidence that melatonin blocks insulin secretion and has a potential adverse effect of diabetes in the future [89]. Serum melatonin level in patients with OSA is abnormally low [90], but no consensus exists whether melatonin supplementation may be beneficial in T2DM patients with OSA.
As obesity is the critical risk factor for OSA and diabetes, weight reduction attenuates the severity of both diseases. Effective weight loss that is maintained has lasting beneficial effects on energy metabolism, and helps to prevent adverse metabolic and cardiovascular events [91]. Lifestyle modification in patients with OSA and diabetes has multiple benefits including weight reduction and improvements in both severity of OSA and glucose control [92].
Continuous positive airway pressure (CPAP) is the first line treatment of OSA. However, it is not clear whether it has a favorable effect on glucose metabolism [939495]. Some studies failed to show improvement of insulin sensitivity assessed by both homeostasis model assessment and euglycaemic hyperinsulinaemic clamp in OSA patients that used CPAP for about 5 hours a day over up to 3 months [96979899]. Yet other studies demonstrated improved insulin sensitivity and glucose control after CPAP in obese patients with severe OSA [100101].
Contrasting study results may stem from limitations with CPAP adherence and treatment duration. One study showed increased insulin sensitivity at 24 weeks but not at 12 weeks, which suggest the importance of longer exposure time to CPAP [102]. The influence of REM sleep which predominantly occurs during the second half of the sleep period is also important to consider, in that OSA related respiratory events are more severe secondary to REM-related muscle atonia [103]. Poor CPAP adherence will often result in lack of adequate treatment during the later portion of the sleep period since therapy is most frequently stopped a few hours into the sleep period, resulting in greater patient exposure to REM-related OSA.
CPAP will not only prevent hypoxic events, but also reduce arousals and nocturnal sympathetic activity, ultimately leading to improved sleep continuity [104]. Potential adverse metabolic effects of OSA such as higher levels of inflammatory cytokines and leptin may also be decreased by CPAP [105106107]. Presumably, based on mechanisms described above, the end effect is improved glycemic control. However, based on available data, this still remains debatable.
The relationship between OSA and diabetes is bidirectional. OSA induced intermittent hypoxia and arousals may result in decreased insulin sensitivity, sympathetic excitation, and systemic inflammation that eventually lead to diabetes. At the same time, uncontrolled glucose may desensitize the carotid body and pharyngeal dilator muscle that promote sleep disordered breathing in OSA. Obesity and diabetic neuropathy may worsen OSA disease severity.
Treatment of OSA with melatonin lacks evidence, whereas, lifestyle modification with weight loss is strongly recommended for the management of OSA and diabetes. Treatment with CPAP is useful as it remains first-line therapy for OSA and may stabilize other metabolic conditions resulting in improved glycemic control. Further research is needed to better understand the potential bi-directional associations between diabetes and OSA, which hopefully will help lead to the development of more effective prevention and treatment interventions.
References
1. Burwell CS, Robin ED, Whaley RD, Bickelmann AG. Extreme obesity associated with alveolar hypoventilation: a Pickwickian syndrome 1956. Obes Res. 1994; 2:390–397. PMID: 16353591.
2. American Academy of Sleep Medicine. International classification of sleep disorders. 3rd ed. Darien: American Academy of Sleep Medicine;2014.
3. Eckert DJ, White DP, Jordan AS, Malhotra A, Wellman A. Defining phenotypic causes of obstructive sleep apnea. Identification of novel therapeutic targets. Am J Respir Crit Care Med. 2013; 188:996–1004. PMID: 23721582.

4. Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993; 328:1230–1235. PMID: 8464434.

5. Narkiewicz K, Somers VK. Sympathetic nerve activity in obstructive sleep apnoea. Acta Physiol Scand. 2003; 177:385–390. PMID: 12609010.

6. American Academy of Sleep Medicine. International classification of sleep disorders: diagnostic and coding manual. 3rd ed. Darien: American Academy of Sleep Medicine;2014.
7. Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008; 5:136–143. PMID: 18250205.

8. Heinzer R, Vat S, Marques-Vidal P, Marti-Soler H, Andries D, Tobback N, Mooser V, Preisig M, Malhotra A, Waeber G, Vollenweider P, Tafti M, Haba-Rubio J. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med. 2015; 3:310–318. PMID: 25682233.

9. Elmasry A, Lindberg E, Berne C, Janson C, Gislason T, Awad Tageldin M, Boman G. Sleep-disordered breathing and glucose metabolism in hypertensive men: a population-based study. J Intern Med. 2001; 249:153–161. PMID: 11240844.

10. Ip MS, Lam B, Ng MM, Lam WK, Tsang KW, Lam KS. Obstructive sleep apnea is independently associated with insulin resistance. Am J Respir Crit Care Med. 2002; 165:670–676. PMID: 11874812.

11. Duran J, Esnaola S, Rubio R, Iztueta A. Obstructive sleep apnea-hypopnea and related clinical features in a population-based sample of subjects aged 30 to 70 yr. Am J Respir Crit Care Med. 2001; 163(3 Pt 1):685–689. PMID: 11254524.

12. Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013; 177:1006–1014. PMID: 23589584.

13. Udwadia ZF, Doshi AV, Lonkar SG, Singh CI. Prevalence of sleep-disordered breathing and sleep apnea in middle-aged urban Indian men. Am J Respir Crit Care Med. 2004; 169:168–173. PMID: 14604837.

14. Bixler EO, Vgontzas AN, Ten Have T, Tyson K, Kales A. Effects of age on sleep apnea in men: I. Prevalence and severity. Am J Respir Crit Care Med. 1998; 157:144–148. PMID: 9445292.
15. Kim J, In K, Kim J, You S, Kang K, Shim J, Lee S, Lee J, Lee S, Park C, Shin C. Prevalence of sleep-disordered breathing in middle-aged Korean men and women. Am J Respir Crit Care Med. 2004; 170:1108–1113. PMID: 15347562.

16. Foster GD, Sanders MH, Millman R, Zammit G, Borradaile KE, Newman AB, Wadden TA, Kelley D, Wing RR, Sunyer FX, Darcey V, Kuna ST. Sleep AHEAD Research Group. Obstructive sleep apnea among obese patients with type 2 diabetes. Diabetes Care. 2009; 32:1017–1019. PMID: 19279303.

17. Borel AL, Benhamou PY, Baguet JP, Halimi S, Levy P, Mallion JM, Pepin JL. High prevalence of obstructive sleep apnoea syndrome in a type 1 diabetic adult population: a pilot study. Diabet Med. 2010; 27:1328–1329. PMID: 20950392.
18. Schober AK, Neurath MF, Harsch IA. Prevalence of sleep apnoea in diabetic patients. Clin Respir J. 2011; 5:165–172. PMID: 21679352.

19. Manin G, Pons A, Baltzinger P, Moreau F, Iamandi C, Wilhelm JM, Lenoble P, Kessler L, Kessler R. Obstructive sleep apnoea in people with type 1 diabetes: prevalence and association with micro- and macrovascular complications. Diabet Med. 2015; 32:90–96. PMID: 25186832.
20. Punjabi NM, Shahar E, Redline S, Gottlieb DJ, Givelber R, Resnick HE. Sleep Heart Health Study Investigators. Sleep-disordered breathing, glucose intolerance, and insulin resistance: the Sleep Heart Health Study. Am J Epidemiol. 2004; 160:521–530. PMID: 15353412.

21. Reutrakul S, Van Cauter E. Interactions between sleep, circadian function, and glucose metabolism: implications for risk and severity of diabetes. Ann N Y Acad Sci. 2014; 1311:151–173. PMID: 24628249.

22. Ryan S. Adipose tissue inflammation by intermittent hypoxia: mechanistic link between obstructive sleep apnoea and metabolic dysfunction. J Physiol. 2017; 595:2423–2430. PMID: 27901270.

23. Narkiewicz K, van de Borne PJ, Montano N, Dyken ME, Phillips BG, Somers VK. Contribution of tonic chemoreflex activation to sympathetic activity and blood pressure in patients with obstructive sleep apnea. Circulation. 1998; 97:943–945. PMID: 9529260.

24. Reichmuth KJ, Austin D, Skatrud JB, Young T. Association of sleep apnea and type II diabetes: a population-based study. Am J Respir Crit Care Med. 2005; 172:1590–1595. PMID: 16192452.
25. Penev PD. Update on energy homeostasis and insufficient sleep. J Clin Endocrinol Metab. 2012; 97:1792–1801. PMID: 22442266.

26. Shechter A. Obstructive sleep apnea and energy balance regulation: a systematic review. Sleep Med Rev. 2017; 34:59–69. PMID: 27818084.

27. Punjabi NM, Sorkin JD, Katzel LI, Goldberg AP, Schwartz AR, Smith PL. Sleep-disordered breathing and insulin resistance in middle-aged and overweight men. Am J Respir Crit Care Med. 2002; 165:677–682. PMID: 11874813.

28. Punjabi NM, Beamer BA. Alterations in glucose disposal in sleep-disordered breathing. Am J Respir Crit Care Med. 2009; 179:235–240. PMID: 19011148.

29. Hjalmarsen A, Aasebo U, Birkeland K, Sager G, Jorde R. Impaired glucose tolerance in patients with chronic hypoxic pulmonary disease. Diabetes Metab. 1996; 22:37–42. PMID: 8697294.
30. Larsen JJ, Hansen JM, Olsen NV, Galbo H, Dela F. The effect of altitude hypoxia on glucose homeostasis in men. J Physiol. 1997; 504:241–249. PMID: 9350634.

31. Newhouse LP, Joyner MJ, Curry TB, Laurenti MC, Man CD, Cobelli C, Vella A, Limberg JK. Three hours of intermittent hypoxia increases circulating glucose levels in healthy adults. Physiol Rep. 2017; 5:e13106. PMID: 28087818.

32. Andrews RC, Walker BR. Glucocorticoids and insulin resistance: old hormones, new targets. Clin Sci (Lond). 1999; 96:513–523. PMID: 10209084.

33. Coste O, Beers PV, Bogdan A, Charbuy H, Touitou Y. Hypoxic alterations of cortisol circadian rhythm in man after simulation of a long duration flight. Steroids. 2005; 70:803–810. PMID: 16019044.

34. Anand IS, Chandrashekhar Y, Rao SK, Malhotra RM, Ferrari R, Chandana J, Ramesh B, Shetty KJ, Boparai MS. Body fluid compartments, renal blood flow, and hormones at 6,000 m in normal subjects. J Appl Physiol (1985). 1993; 74:1234–1239. PMID: 8482663.

35. Saha S, Schwarz PE, Bergmann S, Bornstein SR, Graessler J, Kopprasch S. Circulating very-low-density lipoprotein from subjects with impaired glucose tolerance accelerates adrenocortical cortisol and aldosterone synthesis. Horm Metab Res. 2013; 45:169–172. PMID: 23047828.

36. Narkiewicz K, van de Borne PJ, Pesek CA, Dyken ME, Montano N, Somers VK. Selective potentiation of peripheral chemoreflex sensitivity in obstructive sleep apnea. Circulation. 1999; 99:1183–1189. PMID: 10069786.

37. Deibert DC, DeFronzo RA. Epinephrine-induced insulin resistance in man. J Clin Invest. 1980; 65:717–721. PMID: 6243677.

38. Ohga E, Nagase T, Tomita T, Teramoto S, Matsuse T, Katayama H, Ouchi Y. Increased levels of circulating ICAM-1, VCAM-1, and L-selectin in obstructive sleep apnea syndrome. J Appl Physiol (1985). 1999; 87:10–14. PMID: 10409552.
39. Alberti A, Sarchielli P, Gallinella E, Floridi A, Floridi A, Mazzotta G, Gallai V. Plasma cytokine levels in patients with obstructive sleep apnea syndrome: a preliminary study. J Sleep Res. 2003; 12:305–311. PMID: 14633242.

40. El-Solh AA, Mador MJ, Sikka P, Dhillon RS, Amsterdam D, Grant BJ. Adhesion molecules in patients with coronary artery disease and moderate-to-severe obstructive sleep apnea. Chest. 2002; 121:1541–1547. PMID: 12006441.

41. Lee EJ, Heo W, Kim JY, Kim H, Kang MJ, Kim BR, Kim JH, Park DY, Kim CH, Yoon JH, Cho HJ. Alteration of inflammatory mediators in the upper and lower airways under chronic intermittent hypoxia: preliminary animal study. Mediators Inflamm. 2017; 2017:4327237. PMID: 29038619.

42. Wieser V, Moschen AR, Tilg H. Inflammation, cytokines and insulin resistance: a clinical perspective. Arch Immunol Ther Exp (Warsz). 2013; 61:119–125. PMID: 23307037.

43. Tasali E, Leproult R, Ehrmann DA, Van Cauter E. Slow-wave sleep and the risk of type 2 diabetes in humans. Proc Natl Acad Sci U S A. 2008; 105:1044–1049. PMID: 18172212.

44. Knutson KL, Ryden AM, Mander BA, Van Cauter E. Role of sleep duration and quality in the risk and severity of type 2 diabetes mellitus. Arch Intern Med. 2006; 166:1768–1774. PMID: 16983057.

45. Knutson KL, Van Cauter E, Zee P, Liu K, Lauderdale DS. Cross-sectional associations between measures of sleep and markers of glucose metabolism among subjects with and without diabetes: the Coronary Artery Risk Development in Young Adults (CARDIA) sleep study. Diabetes Care. 2011; 34:1171–1176. PMID: 21411507.
46. Loredo JS, Ziegler MG, Ancoli-Israel S, Clausen JL, Dimsdale JE. Relationship of arousals from sleep to sympathetic nervous system activity and BP in obstructive sleep apnea. Chest. 1999; 116:655–659. PMID: 10492267.

47. Buxton OM, Pavlova M, Reid EW, Wang W, Simonson DC, Adler GK. Sleep restriction for 1 week reduces insulin sensitivity in healthy men. Diabetes. 2010; 59:2126–2133. PMID: 20585000.

48. Irwin M, Thompson J, Miller C, Gillin JC, Ziegler M. Effects of sleep and sleep deprivation on catecholamine and interleukin-2 levels in humans: clinical implications. J Clin Endocrinol Metab. 1999; 84:1979–1985. PMID: 10372697.

49. Berti L, Kellerer M, Capp E, Haring HU. Leptin stimulates glucose transport and glycogen synthesis in C2C12 myotubes: evidence for a P13-kinase mediated effect. Diabetologia. 1997; 40:606–609. PMID: 9165231.
50. Taheri S, Lin L, Austin D, Young T, Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004; 1:e62. PMID: 15602591.

51. Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999; 354:1435–1439. PMID: 10543671.

52. Leproult R, Copinschi G, Buxton O, Van Cauter E. Sleep loss results in an elevation of cortisol levels the next evening. Sleep. 1997; 20:865–870. PMID: 9415946.
53. Holliday EG, Magee CA, Kritharides L, Banks E, Attia J. Short sleep duration is associated with risk of future diabetes but not cardiovascular disease: a prospective study and meta-analysis. PLoS One. 2013; 8:e82305. PMID: 24282622.

54. Hastings MH, Maywood ES, Brancaccio M. Generation of circadian rhythms in the suprachiasmatic nucleus. Nat Rev Neurosci. 2018; 19:453–469. PMID: 29934559.

55. Stawerska R, Smyczynska J, Hilczer M, Lewinski A. Changes in circadian rhythm of prolactin in short children are dependent on growth hormone secretion. Ann Agric Environ Med. 2014; 21:445–449. PMID: 24959807.
56. Huang W, Ramsey KM, Marcheva B, Bass J. Circadian rhythms, sleep, and metabolism. J Clin Invest. 2011; 121:2133–2141. PMID: 21633182.

57. Leproult R, Holmback U, Van Cauter E. Circadian misalignment augments markers of insulin resistance and inflammation, independently of sleep loss. Diabetes. 2014; 63:1860–1869. PMID: 24458353.

58. Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci U S A. 2009; 106:4453–4458. PMID: 19255424.

59. Nakao T, Kohsaka A, Otsuka T, Thein ZL, Le HT, Waki H, Gouraud SS, Ihara H, Nakanishi M, Sato F, Muragaki Y, Maeda M. Impact of heart-specific disruption of the circadian clock on systemic glucose metabolism in mice. Chronobiol Int. 2018; 35:499–510. PMID: 29271671.

60. Vieira E, Burris TP, Quesada I. Clock genes, pancreatic function, and diabetes. Trends Mol Med. 2014; 20:685–693. PMID: 25457619.

61. Reutrakul S, Siwasaranond N, Nimitphong H, Saetung S, Chirakalwasan N, Chailurkit LO, Srijaruskul K, Ongphiphadhanakul B, Thakkinstian A. Associations between nocturnal urinary 6-sulfatoxymelatonin, obstructive sleep apnea severity and glycemic control in type 2 diabetes. Chronobiol Int. 2017; 34:382–392. PMID: 28128991.

62. Peschke E, Muhlbauer E. New evidence for a role of melatonin in glucose regulation. Best Pract Res Clin Endocrinol Metab. 2010; 24:829–841. PMID: 21112029.

63. Peschke E, Frese T, Chankiewitz E, Peschke D, Preiss U, Schneyer U, Spessert R, Muhlbauer E. Diabetic Goto Kakizaki rats as well as type 2 diabetic patients show a decreased diurnal serum melatonin level and an increased pancreatic melatonin-receptor status. J Pineal Res. 2006; 40:135–143. PMID: 16441550.

64. McMullan CJ, Schernhammer ES, Rimm EB, Hu FB, Forman JP. Melatonin secretion and the incidence of type 2 diabetes. JAMA. 2013; 309:1388–1396. PMID: 23549584.

65. Horner RL, Brooks D, Kozar LF, Tse S, Phillipson EA. Immediate effects of arousal from sleep on cardiac autonomic outflow in the absence of breathing in dogs. J Appl Physiol (1985). 1995; 79:151–162. PMID: 7559214.

66. Nonogaki K. New insights into sympathetic regulation of glucose and fat metabolism. Diabetologia. 2000; 43:533–549. PMID: 10855527.

67. Avogaro A, Toffolo G, Valerio A, Cobelli C. Epinephrine exerts opposite effects on peripheral glucose disposal and glucose-stimulated insulin secretion: a stable label intravenous glucose tolerance test minimal model study. Diabetes. 1996; 45:1373–1378. PMID: 8826974.

68. Raz I, Katz A, Spencer MK. Epinephrine inhibits insulin-mediated glycogenesis but enhances glycolysis in human skeletal muscle. Am J Physiol. 1991; 260:E430–E435. PMID: 1900669.

69. Roden M, Price TB, Perseghin G, Petersen KF, Rothman DL, Cline GW, Shulman GI. Mechanism of free fatty acid-induced insulin resistance in humans. J Clin Invest. 1996; 97:2859–2865. PMID: 8675698.

70. Santomauro AT, Boden G, Silva ME, Rocha DM, Santos RF, Ursich MJ, Strassmann PG, Wajchenberg BL. Overnight lowering of free fatty acids with Acipimox improves insulin resistance and glucose tolerance in obese diabetic and nondiabetic subjects. Diabetes. 1999; 48:1836–1841. PMID: 10480616.

71. Hucking K, Hamilton-Wessler M, Ellmerer M, Bergman RN. Burst-like control of lipolysis by the sympathetic nervous system in vivo. J Clin Invest. 2003; 111:257–264. PMID: 12531882.

72. Julius S, Gudbrandsson T, Jamerson K, Andersson O. The interconnection between sympathetics, microcirculation, and insulin resistance in hypertension. Blood Press. 1992; 1:9–19. PMID: 1345145.

73. Jamerson KA, Julius S, Gudbrandsson T, Andersson O, Brant DO. Reflex sympathetic activation induces acute insulin resistance in the human forearm. Hypertension. 1993; 21:618–623. PMID: 8491496.

74. Klein J, Fasshauer M, Ito M, Lowell BB, Benito M, Kahn CR. Beta(3)-adrenergic stimulation differentially inhibits insulin signaling and decreases insulin-induced glucose uptake in brown adipocytes. J Biol Chem. 1999; 274:34795–34802. PMID: 10574950.
75. Mondini S, Guilleminault C. Abnormal breathing patterns during sleep in diabetes. Ann Neurol. 1985; 17:391–395. PMID: 4004160.

76. Kadoglou NP, Avgerinos ED, Liapis CD. An update on markers of carotid atherosclerosis in patients with type 2 diabetes. Biomark Med. 2010; 4:601–609. PMID: 20701448.

77. Alvarez-Buylla R, de Alvarez-Buylla ER. Carotid sinus receptors participate in glucose homeostasis. Respir Physiol. 1988; 72:347–359. PMID: 3406554.

78. Kline DD, Peng YJ, Manalo DJ, Semenza GL, Prabhakar NR. Defective carotid body function and impaired ventilatory responses to chronic hypoxia in mice partially deficient for hypoxia-inducible factor 1 alpha. Proc Natl Acad Sci U S A. 2002; 99:821–826. PMID: 11792862.
79. Vinik AI, Nevoret ML, Casellini C, Parson H. Diabetic neuropathy. Endocrinol Metab Clin North Am. 2013; 42:747–787. PMID: 24286949.

80. Ficker JH, Dertinger SH, Siegfried W, Konig HJ, Pentz M, Sailer D, Katalinic A, Hahn EG. Obstructive sleep apnoea and diabetes mellitus: the role of cardiovascular autonomic neuropathy. Eur Respir J. 1998; 11:14–19. PMID: 9543264.

81. Bottini P, Redolfi S, Dottorini ML, Tantucci C. Autonomic neuropathy increases the risk of obstructive sleep apnea in obese diabetics. Respiration. 2008; 75:265–271. PMID: 17347559.

82. Rasche K, Keller T, Tautz B, Hader C, Hergenc G, Antosiewicz J, Di Giulio C, Pokorski M. Obstructive sleep apnea and type 2 diabetes. Eur J Med Res. 2010; 15(Suppl 2):152–156.

83. Bottini P, Dottorini ML, Cristina Cordoni M, Casucci G, Tantucci C. Sleep-disordered breathing in nonobese diabetic subjects with autonomic neuropathy. Eur Respir J. 2003; 22:654–660. PMID: 14582920.

84. Tahrani AA, Ali A, Raymond NT, Begum S, Dubb K, Mughal S, Jose B, Piya MK, Barnett AH, Stevens MJ. Obstructive sleep apnea and diabetic neuropathy: a novel association in patients with type 2 diabetes. Am J Respir Crit Care Med. 2012; 186:434–441. PMID: 22723291.
85. Kim AM, Keenan BT, Jackson N, Chan EL, Staley B, Poptani H, Torigian DA, Pack AI, Schwab RJ. Tongue fat and its relationship to obstructive sleep apnea. Sleep. 2014; 37:1639–1648. PMID: 25197815.

86. Auld F, Maschauer EL, Morrison I, Skene DJ, Riha RL. Evidence for the efficacy of melatonin in the treatment of primary adult sleep disorders. Sleep Med Rev. 2017; 34:10–22. PMID: 28648359.

87. Bertuglia S, Reiter RJ. Melatonin reduces microvascular damage and insulin resistance in hamsters due to chronic intermittent hypoxia. J Pineal Res. 2009; 46:307–313. PMID: 19317794.

88. Hung MW, Kravtsov GM, Lau CF, Poon AM, Tipoe GL, Fung ML. Melatonin ameliorates endothelial dysfunction, vascular inflammation, and systemic hypertension in rats with chronic intermittent hypoxia. J Pineal Res. 2013; 55:247–256. PMID: 23869411.

89. Tuomi T, Nagorny CLF, Singh P, Bennet H, Yu Q, Alenkvist I, Isomaa B, Ostman B, Soderstrom J, Pesonen AK, Martikainen S, Raikkonen K, Forsen T, Hakaste L, Almgren P, Storm P, Asplund O, Shcherbina L, Fex M, Fadista J, Tengholm A, Wierup N, Groop L, Mulder H. Increased Melatonin signaling is a risk factor for type 2 diabetes. Cell Metab. 2016; 23:1067–1077. PMID: 27185156.

90. Hernandez C, Abreu J, Abreu P, Castro A, Jimenez A. Nocturnal melatonin plasma levels in patients with OSAS: the effect of CPAP. Eur Respir J. 2007; 30:496–500. PMID: 17537771.

91. Hudgel DW, Patel SR, Ahasic AM, Bartlett SJ, Bessesen DH, Coaker MA, Fiander PM, Grunstein RR, Gurubhagavatula I, Kapur VK, Lettieri CJ, Naughton MT, Owens RL, Pepin JD, Tuomilehto H, Wilson KC. American Thoracic Society Assembly on Sleep and Respiratory Neurobiology. The role of weight management in the treatment of adult obstructive sleep apnea. An official American Thoracic Society Clinical Practice Guideline. Am J Respir Crit Care Med. 2018; 198:e70–e87. PMID: 30215551.

92. Kline CE, Burke LE, Sereika SM, Imes CC, Rockette-Wagner B, Mendez DD, Strollo PJ, Zheng Y, Rathbun SL, Chasens ER. Bidirectional relationships between weight change and sleep apnea in a behavioral weight loss intervention. Mayo Clin Proc. 2018; 93:1290–1298. PMID: 30082081.

93. Chakhtoura M, Azar ST. Continuous positive airway pressure and type 2 diabetes mellitus. Diabetes Metab Syndr. 2012; 6:176–179. PMID: 23158985.

94. Punjabi NM. Workshop Participants. Do sleep disorders and associated treatments impact glucose metabolism? Drugs. 2009; 69(Suppl 2):13–27. PMID: 20047348.

95. Chirakalwasan N, Amnakkittikul S, Wanitcharoenkul E, Charoensri S, Saetung S, Chanprasertyothin S, Chailurkit LO, Panburana P, Bumrungphuet S, Thakkinstian A, Reutrakul S. Continuous positive airway pressure therapy in gestational diabetes with obstructive sleep apnea: a randomized controlled trial. J Clin Sleep Med. 2018; 14:327–336. PMID: 29458699.

96. West SD, Nicoll DJ, Wallace TM, Matthews DR, Stradling JR. Effect of CPAP on insulin resistance and HbA1c in men with obstructive sleep apnoea and type 2 diabetes. Thorax. 2007; 62:969–974. PMID: 17557769.

97. Kohler M, Stoewhas AC, Ayers L, Senn O, Bloch KE, Russi EW, Stradling JR. Effects of continuous positive airway pressure therapy withdrawal in patients with obstructive sleep apnea: a randomized controlled trial. Am J Respir Crit Care Med. 2011; 184:1192–1199. PMID: 21836134.
98. Sivam S, Phillips CL, Trenell MI, Yee BJ, Liu PY, Wong KK, Grunstein RR. Effects of 8 weeks of continuous positive airway pressure on abdominal adiposity in obstructive sleep apnoea. Eur Respir J. 2012; 40:913–918. PMID: 22267762.

99. Hecht L, Mohler R, Meyer G. Effects of CPAP-respiration on markers of glucose metabolism in patients with obstructive sleep apnoea syndrome: a systematic review and meta-analysis. Ger Med Sci. 2011; 9:Doc20. PMID: 21863134.
100. Shaw JE, Punjabi NM, Naughton MT, Willes L, Bergenstal RM, Cistulli PA, Fulcher GR, Richards GN, Zimmet PZ. The effect of treatment of obstructive sleep apnea on glycemic control in type 2 diabetes. Am J Respir Crit Care Med. 2016; 194:486–492. PMID: 26926656.

101. Lam JC, Lam B, Yao TJ, Lai AY, Ooi CG, Tam S, Lam KS, Ip MS. A randomized controlled trial of nasal continuous positive airway pressure on insulin sensitivity in obstructive sleep apnoea. Eur Respir J. 2010; 35:138–145. PMID: 19608589.
102. Hoyos CM, Killick R, Yee BJ, Phillips CL, Grunstein RR, Liu PY. Cardiometabolic changes after continuous positive airway pressure for obstructive sleep apnoea: a randomised sham-controlled study. Thorax. 2012; 67:1081–1089. PMID: 22561530.

103. Fraigne JJ, Grace KP, Horner RL, Peever J. Mechanisms of REM sleep in health and disease. Curr Opin Pulm Med. 2014; 20:527–532. PMID: 25221856.

104. Marrone O, Salvaggio A, Bue AL, Bonanno A, Riccobono L, Insalaco G, Bonsignore MR. Blood pressure changes after automatic and fixed CPAP in obstructive sleep apnea: relationship with nocturnal sympathetic activity. Clin Exp Hypertens. 2011; 33:373–380. PMID: 21529314.

105. Saarelainen S, Lahtela J, Kallonen E. Effect of nasal CPAP treatment on insulin sensitivity and plasma leptin. J Sleep Res. 1997; 6:146–147. PMID: 9377535.

106. Harsch IA, Konturek PC, Koebnick C, Kuehnlein PP, Fuchs FS, Pour Schahin S, Wiest GH, Hahn EG, Lohmann T, Ficker JH. Leptin and ghrelin levels in patients with obstructive sleep apnoea: effect of CPAP treatment. Eur Respir J. 2003; 22:251–257. PMID: 12952256.

107. Dyugovskaya L, Lavie P, Lavie L. Lymphocyte activation as a possible measure of atherosclerotic risk in patients with sleep apnea. Ann N Y Acad Sci. 2005; 1051:340–350. PMID: 16126976.

Fig. 1
Proposed interaction of obstructive sleep apnea (OSA) and diabetes. RERA, respiratory effort related arousal; DM, diabetes mellitus.
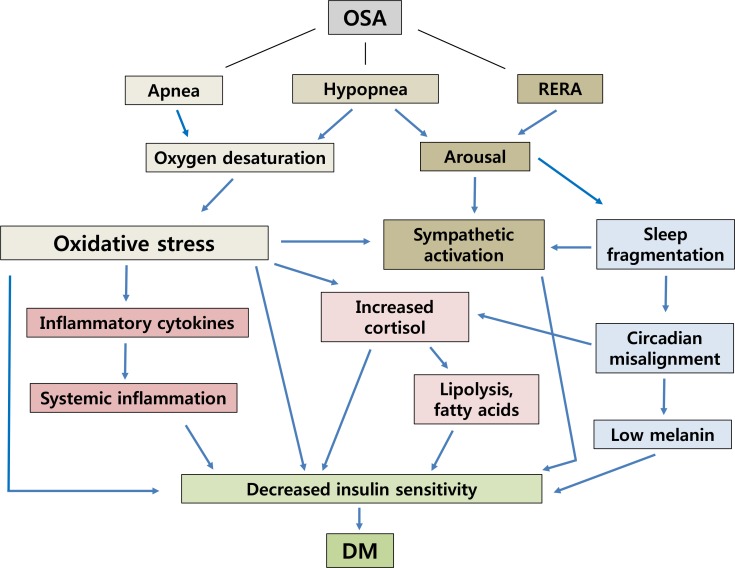
Table 1
Diagnostic criteria for obstructive sleep apnea: (A and B) or C satisfy the criteria
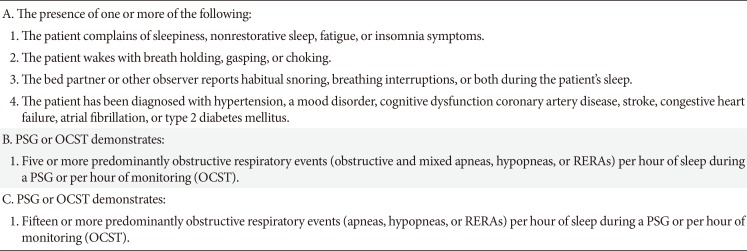
Table 2
OSA prevalence
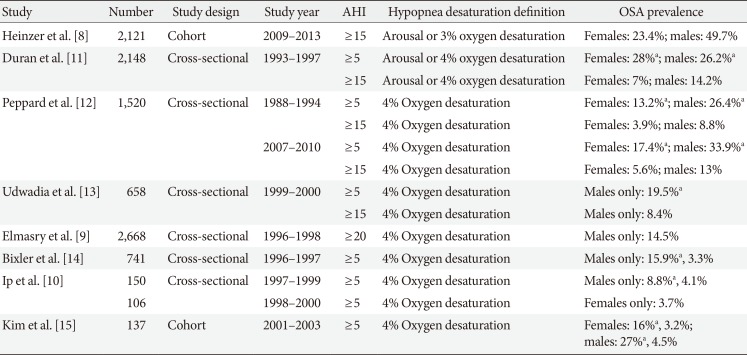
| Study | Number | Study design | Study year | AHI | Hypopnea desaturation definition | OSA prevalence |
|---|---|---|---|---|---|---|
| Heinzer et al. [8] | 2,121 | Cohort | 2009–2013 | ≥15 | Arousal or 3% oxygen desaturation | Females: 23.4%; males: 49.7% |
| Duran et al. [11] | 2,148 | Cross-sectional | 1993–1997 | ≥5 | Arousal or 4% oxygen desaturation | Females: 28%a; males: 26.2%a |
| ≥15 | Arousal or 4% oxygen desaturation | Females: 7%; males: 14.2% | ||||
| Peppard et al. [12] | 1,520 | Cross-sectional | 1988–1994 | ≥5 | 4% Oxygen desaturation | Females: 13.2%a; males: 26.4%a |
| ≥15 | 4% Oxygen desaturation | Females: 3.9%; males: 8.8% | ||||
| 2007–2010 | ≥5 | 4% Oxygen desaturation | Females: 17.4%a; males: 33.9%a | |||
| ≥15 | 4% Oxygen desaturation | Females: 5.6%; males: 13% | ||||
| Udwadia et al. [13] | 658 | Cross-sectional | 1999–2000 | ≥5 | 4% Oxygen desaturation | Males only: 19.5%a |
| ≥15 | 4% Oxygen desaturation | Males only: 8.4% | ||||
| Elmasry et al. [9] | 2,668 | Cross-sectional | 1996–1998 | ≥20 | 4% Oxygen desaturation | Males only: 14.5% |
| Bixler et al. [14] | 741 | Cross-sectional | 1996–1997 | ≥5 | 4% Oxygen desaturation | Males only: 15.9%a, 3.3% |
| Ip et al. [10] | 150 | Cross-sectional | 1997–1999 | ≥5 | 4% Oxygen desaturation | Males only: 8.8%a, 4.1% |
| 106 | 1998–2000 | ≥5 | 4% Oxygen desaturation | Females only: 3.7% | ||
| Kim et al. [15] | 137 | Cohort | 2001–2003 | ≥5 | 4% Oxygen desaturation | Females: 16%a, 3.2%; males: 27%a, 4.5% |




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download