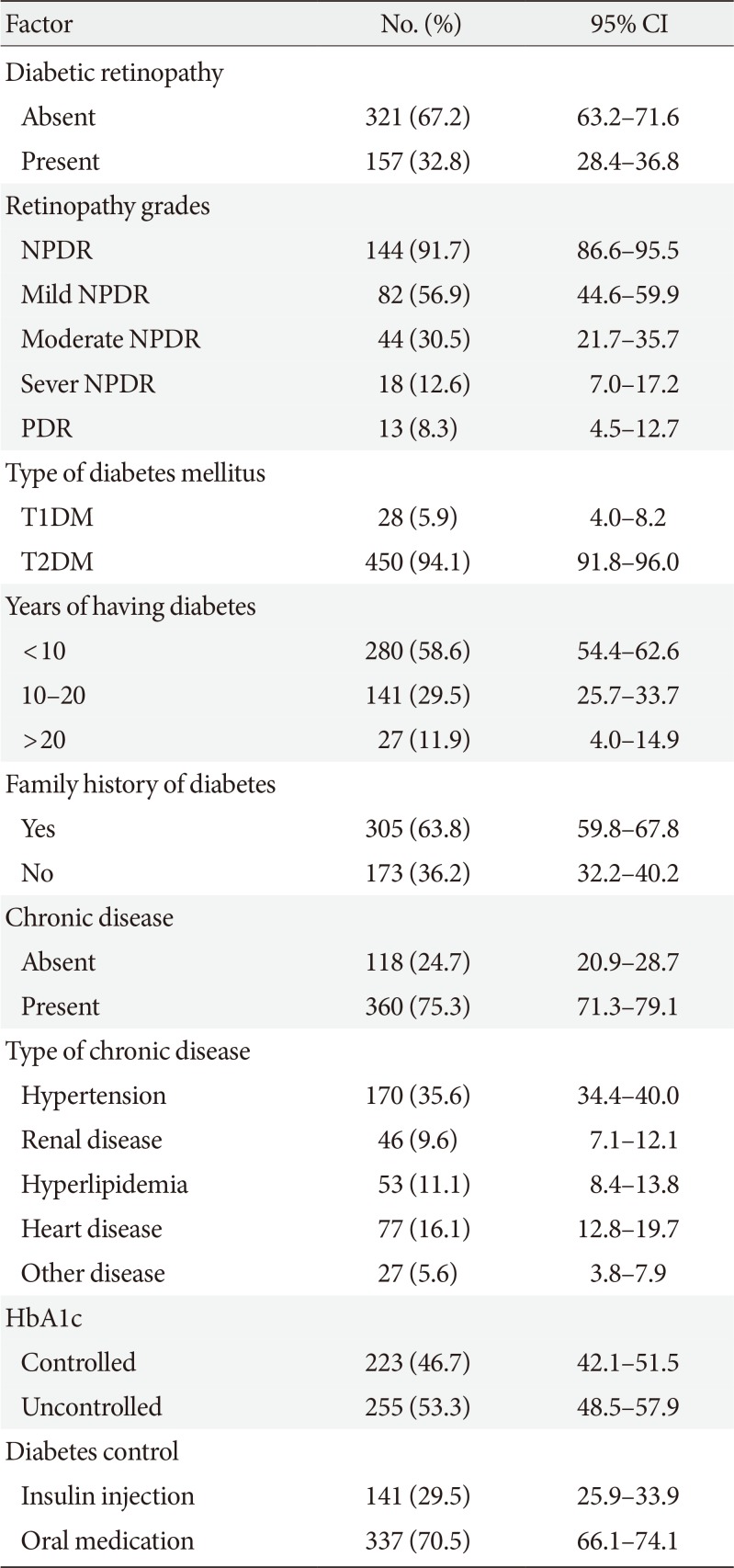
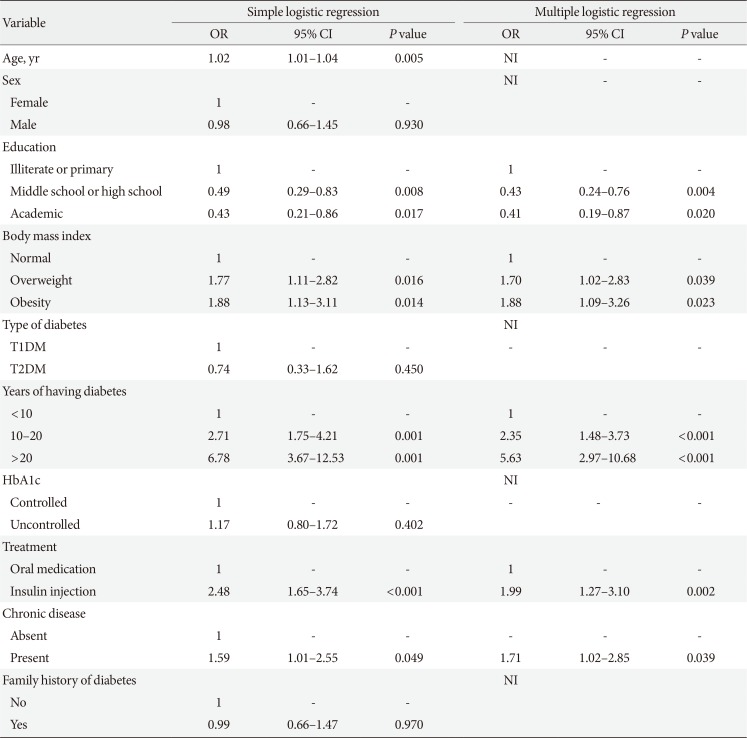
The study findings showed that the prevalence of DR was 32.8% among diabetic patients. In addition, the results of multivariable regression analysis revealed a significant relationship between DR and diabetes duration, BMI, type of diabetes treatment, education level, and chronic diseases.
The prevalence of DR in the present study was different from or lower than that reported in other studies. For instance, in Armenia the prevalence of DR was 36.2% [
13], in Nepal was 44.7% [
8], and 37% in another region of Iran [
5]. Also, this rate was 11.9% in China [
7] and 19% in United Arab Emirates [
4]. Generally, many studies have reported different rates of DR, with the estimates largely depending on methodology, diagnostic methods, effect of interventional factors, and study samples [
513]. Overweight and obesity as major complications associated with diabetes have become a major public health problem worldwide as they have shown an increasing trend in many countries [
7]. Therefore, BMI should be taken into account as a major risk factor associated with DR in studies focusing on diabetes and its related complications, such as DR. The present study showed that BMI was positively associated with DR development. Accordingly, overweight and obese patients were respectively 1.70 and 1.88 times more likely to develop DR than those with normal BMI [
14]. However, this was not consistent with the results of several studies [
1516]. In addition, duration of diabetes was positively associated with DR, which is in line with the previous studies [
17]. Our findings showed that the patients with higher education levels were less likely to develop DR than illiterate patients. A previous study also examined the relationship between health literacy and diabetes outcomes. The results revealed that participants with lower education levels were at a higher risk of DR [
18]. However, the findings of our study were not in agreement with some other studies [
1419]. Our results showed that the risk of DR was significantly higher (1.71 times) in diabetic patients with chronic diseases compared to those with no chronic diseases. Javadi et al. [
5] reported that the prevalence of DR was 1.55 times higher in diabetic patients with hypertension. They also found a positive, but not statistically significant association between hyperlipidemia and DR [
5]. Moreover, a study in Nepal showed that diabetic patients with hypertension were 2.41 times more likely to develop DR [
8]. Several studies have also shown a significant association between heart diseases and DR in diabetic patients [
15]. However, some studies have indicated no significant associations between the presence of chronic diseases and DR [
1314]. The present study showed a higher risk of DR among patients who were treated with insulin in comparison to those treated with oral medications; hence, our result was in line with the results of the previous studies [
1517]. Although age was a risk factor associated with DR in univariate analysis, it was considered as a confounding factor in multivariable analysis. Many studies have supported these findings, but have mentioned that age is not significant [
1720]. Nonetheless, few studies have disclosed age as a risk factor for DR [
8]. The present study revealed that type of diabetes was not significantly associated with DR, which is inconsistent with some studies [
513]. However, some other studies have shown a significant association between type of diabetes and DR [
46]. Moreover, patients with controlled and uncontrolled HbA1c levels were not significantly different regarding the risk of DR. Furthermore, family history of diabetes was not significantly associated with DR, which is similar to the previous study [
4]. Finally, the prevalence of DR was 32.8% in this study. Given the inconsistency in the results of the previous studies, as well as DR prevalence it seems that policymakers and healthcare providers haven't been able to come up with an applicable strategy.
In conclusion, our study indicated that longer diabetes duration and obesity or having chronic disease are strongly associated with DR suggesting that control of these risk factors may reduce both the prevalence and impact of retinopathy in Iranian patients.
Recruiting participants who had visited the biggest referral center in Southern Iran makes our results generalizable for the population of Shiraz. We selected a major referral center in Southern Iran, and it seems that there are no significant differences between demographic and clinical characteristics of recruited diabetic patients and not-recruited patients who visited the center. However, there is the possibility that clinical characteristics between recruited and not-recruited diabetic patients may be different. Authors checked patients' medical records, but due to lack of comprehensive data registry, some biochemical data were not available.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download