INTRODUCTION
The increasing prevalence of diabetes has led to a proportionate increase in the incidence of diabetic foot ulcers (DFUs) [
1]. DFU is a foot condition in patients with diabetes in which a combined pathology of peripheral motor and sensory neuropathy, autonomic dysfunction, foot deformity, uneven biomechanical loading of the foot, and minor trauma, in conjunction with pre-existing peripheral vascular disease, leads to foot ulceration [
234]. When complicated by ischemia and/or infection, these ulcerations result in foot necrosis that may eventually require amputation. Diabetes-related lower extremity amputations (LEAs) are not only costly, but also physically and psychologically disabling to the affected individual [
25]. They also pose a significant preventable burden to the healthcare system [
3]. Measures to control the severity of DFU and reduce the incidence of LEAs are increasingly being recognized as an important indicator of quality of diabetes care [
2367].
In recent years, more institutions worldwide are adopting a multidisciplinary approach to diabetic foot care [
3]. This approach offers an integrated treatment protocol based on the consensus from various specialties involved in the care of DFU, including endocrinology, podiatric surgery, and vascular intervention. Through this system, many studies have demonstrated improved glycemic control and promising LEA outcomes in terms of reduced major LEAs [
8910].
Currently in Korea, although there are many established multidisciplinary diabetic foot teams, there is a lack of epidemiologic data regarding on either the incidence of DFU or the outcomes of a multidisciplinary approach. This study aims to describe the trends of DFU over a 10-year period at a single tertiary referral center in Korea, and to examine treatment outcomes both before and after the introduction of a multidisciplinary diabetic foot team.
METHODS
Subjects and data collection
In this retrospective cross sectional study, 390 patients with DFU were identified by searching for International Classification of Diseases, 9th revision (ICD-9) codes related to DFU or diabetes-related LEA in diabetic patients who were admitted for DFU to the Yeungnam University Medical Center, Daegu, Republic of Korea, from January 1, 2002, to December 31, 2015. Medical records, including admission notes, discharge summary notes, and consultation notes were reviewed to verify the primary intent of admission. Exclusion criteria were patient cases with missing or incomplete data (n=20), multiple admissions, in which case the data from the first admission was included (n=28), traumatic LEA (n=3), or neoplastic cause of DFU (n=1). After exclusion, the final study population consisted of 338 patients with DFU.
Patients were divided into two groups based on their year of first admission. Year 2012 was chosen as the cutoff point because it was the year when the multidisciplinary diabetic foot team initiated. Patients first admitted before the end of year 2011 were allocated to group A, while patients first admitted after the beginning of year 2012 were allocated to group B. As mentioned earlier, in patient cases with multiple admission records, most of the data was sourced from the first admission, except for surgical records from a later admission but within the study period.
The primary outcome of this study was in the difference in proportion of major LEAs, minor LEAs, and length of hospital stay. The secondary outcome was in the difference in clinical parameters and proportion of peripheral revascularization.
This study was approved by the Institutional Review Board of Yeungnam University Hospital (IRB No. 2018-04-020). This study followed the Declaration of Helsinki on medical protocol and ethics, and throughout the data collection process, all patient's personal information was blinded to researchers. Due to the retrospective nature of this study, the requirement for written informed consent was waived.
Clinical survey and diabetic complications studies
Participants were diagnosed as having type 2 diabetes mellitus (T2DM) based on a previously documented diagnosis of T2DM or reported use of hypoglycemic agents. The duration of diabetes was defined as the difference in years, as mentioned in records, between the date of first admission and the recorded date of diagnosis of diabetes. Length of hospital stay was defined as the total number of days from admission to discharge. Ankle brachial index (ABI) was defined as the ratio of the systolic blood pressure at the ankle to the systolic blood pressure at the upper arm.
All participants were assessed and examined for the presence of diabetic microvascular and macrovascular complications. The presence of diabetic retinopathy was identified from findings examined by an ophthalmologist. A history of photocoagulation or vitrectomy due to proliferative diabetic retinopathy was also classified as diabetic retinopathy. Diabetic peripheral neuropathy was diagnosed using the Semmes Weinstein monofilament test [
11], clinical score (Michigan Neuropathy Screening Instrument questionnaire [
12]), total symptom score for pain, electrophysiology study, and medical record review. Diabetic nephropathy was defined as urinary albumin-creatinine ratio >30 or estimated glomerular filtration rate <60 mL/min/ 1.73 m
2, calculated using the Modification of Diet in Renal Disease formula: 186×(creatinine)
−1.154×(age)
−0.203×[0.742 (if female)] [
13]. A history of coronary artery disease (CAD) was defined as any history of myocardial infarction, unstable angina, percutaneous transluminal coronary angioplasty, or a coronary artery bypass surgery. A history of cerebrovascular accident was defined as any history of cerebral infarction or transient cerebral ischemia. Hypertension was defined as any previous documented diagnosis of hypertension, or the use of antihypertensive medication.
Classification of diabetic foot ulcer and lower extremity amputation
DFU was defined as a non-neoplastic foot disease in a patient with diabetes where foot ulceration at pressure sites was formed due to a combination of peripheral neuropathy and peripheral vascular disease [
234]. A diabetes-related LEA was defined as an amputation procedure due to DFU-related complications resulting in the surgical removal of bones by the transverse anatomical plane of any part of the lower limb. A major LEA was defined as an LEA procedure above the ankle level, while a minor LEA was defined as an LEA procedure below the ankle level. Minor LEA included forefoot amputations (digital, ray), midfoot amputation (transmetatarsal, Lis Frank, Chopart), and hindfoot amputations (Syme). Major LEA included below-knee amputations (transtibial), and above-knee amputations (transfemoral). Knee disarticulation, hip disarticulation cases were not identified throughout the study. In cases with multiple LEAs, the highest level of amputation was considered. Multiple amputation was defined as two or more diabetes-related LEA amputation procedures per patient within the study period.
All patients had their DFU assessed by an endocrinologist at admission. The size of DFU was defined as the longest diameter, in centimeters, from one end of ulcer margin to the other end. The reported cause of DFU was based on records from admission notes. Cases without definite offending event was classified as ‘spontaneous.’ The severity of DFUs was graded according to both the Wagner classification of Diabetic Foot Ulcers and University of Texas (UT) Diabetic Wound Classification System. The Wagner classification organized DFU based on the depth of ulcer and presence of osteomyelitis or gangrene. Grade 0 was assigned to pre-ulcerative lesion. Grade 1 was assigned to partial, superficial ulcer. Grade 2 was assigned to extension into tendon, ligament, fascia, or joint capsule without osteomyelitis. Grade 3 was assigned to deep ulcer with osteomyelitis. Grade 4 was assigned to partial forefoot necrosis. Grade 5 was assigned to extensive foot gangrene. The UT system assessed DFU based on a matrix of ulcer depth on the horizontal axis, and presence of infection or ischemia on the vertical axis. Grade 0 was assigned to ulcerative lesion or healed sites. Grade 1 was assigned to superficial ulcer. Grade 2 was assigned to ulceration with involvement of tendons, capsules, and ligaments. Grade 3 was assigned to ulceration with involvement of either bone or joint. Stage A was assigned to non-ischemic, non-infected wound. Stage B was assigned to non-ischemic, infected wound. Stage C was assigned to ischemic, non-infected wound. Stage D was assigned to ischemic and infected wound [
414].
All patients with complicated DFU (Wagner stage 2 or above) were referred to an orthopedic surgeon for surgical consultation. Decisions regarding the potential utility of surgery and modality of choice, including the level of amputation, were determined by the orthopedic surgeon. Patients who refused surgery despite indications for surgical treatment were managed conservatively.
Patients with ABI lower than 0.9 with or without clinical signs of claudication or critical limb ischemia were consulted to a vascular surgeon for revascularization. Decisions regarding the choice of vascular imaging technique, potential utility of diagnostic angiography and revascularization were made by the vascular surgeon. Patients who refused either diagnostic angiography or revascularization were managed conservatively.
Statistical analysis
All numerical variables in figures and tables were expressed as either mean±standard deviation or numbers with percentages. All statistical analysis except comparison of proportions was performed using SPSS software version 21.0 for Windows (IBM Co., Armonk, NY, USA). Independent two-sample
t-test was used to compare mean values of two continuous variables. Pearson chi-square test was used to compare two proportions from independent samples. A
P<0.05 was considered as statistically significant. Comparison of proportions was analyzed by a free web-based statistical calculator (MedCalc®; MedCalc Software bvba, Ostend, Belgium;
https://www.medcalc.org).
RESULTS
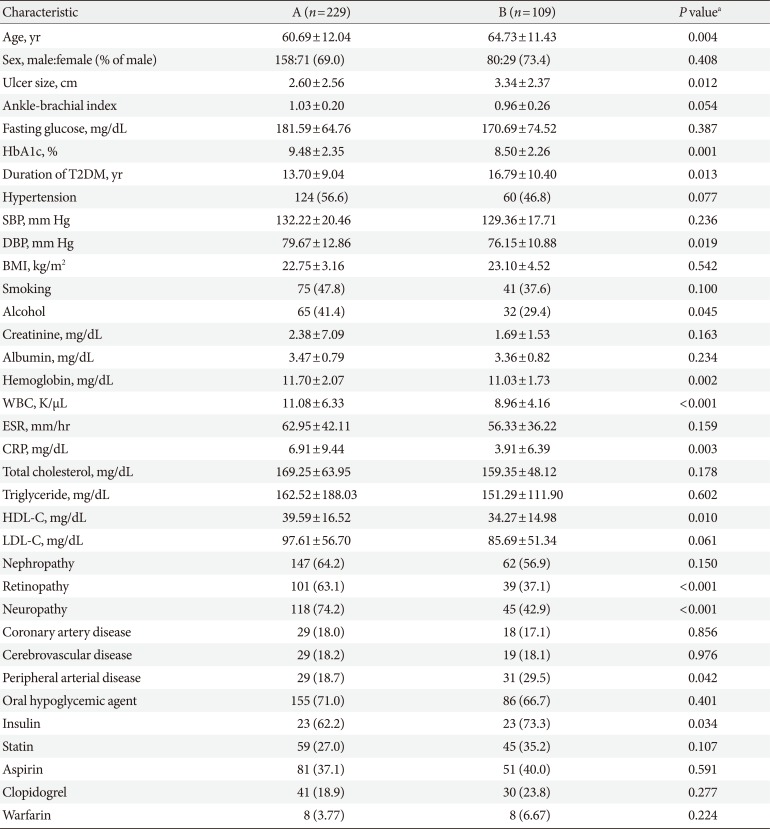
Of the 338 patients from the study period, 229 (about 23 patients/year) were allocated to group A, while 109 (about 26 patients/year) were allocated to group B. The baseline characteristics of both groups are summarized in
Table 1. The mean age of subjects was older for group B (60.69±12.04 years vs. 64.73±11.43 years,
P=0.004). The majority of subjects in both groups were male (69.0% vs. 73.4%,
P=0.408). Glycosylated hemoglobin (HbA1c) was improved (9.48%±2.35% vs. 8.50%±2.26%,
P=0.001), while mean duration of diabetes was longer for group B (13.70±9.04 years vs. 16.79±10.40 years,
P=0.013). The proportion of subjects with hypertension not different (56.6% vs. 46.8%,
P=0.077), and despite comparable systolic blood pressure (132.22±20.46 mm Hg vs. 129.36±17.71 mm Hg,
P=0.236), diastolic blood pressure was lower for group B (79.67±12.86 mm Hg vs. 76.15±10.88 mm Hg,
P=0.019). Mean ulcer size was greater for group B (2.60±2.56 cm vs. 3.34±2.37 cm,
P= 0.012). A decreasing but not significant decreasing trend was observed for ABI (1.03±0.20 vs. 0.96±0.26,
P=0.054).
Table 1
Baseline characteristics of diabetic foot ulcer patients (n=338) at the time of admission between group A (<2012 year) and group B (≥2012 year)
|
Characteristic |
A (n=229) |
B (n=109) |
P valuea
|
|
Age, yr |
60.69±12.04 |
64.73±11.43 |
0.004 |
|
Sex, male:female (% of male) |
158:71 (69.0) |
80:29 (73.4) |
0.408 |
|
Ulcer size, cm |
2.60±2.56 |
3.34±2.37 |
0.012 |
|
Ankle-brachial index |
1.03±0.20 |
0.96±0.26 |
0.054 |
|
Fasting glucose, mg/dL |
181.59±64.76 |
170.69±74.52 |
0.387 |
|
HbA1c, % |
9.48±2.35 |
8.50±2.26 |
0.001 |
|
Duration of T2DM, yr |
13.70±9.04 |
16.79±10.40 |
0.013 |
|
Hypertension |
124 (56.6) |
60 (46.8) |
0.077 |
|
SBP, mm Hg |
132.22±20.46 |
129.36±17.71 |
0.236 |
|
DBP, mm Hg |
79.67±12.86 |
76.15±10.88 |
0.019 |
|
BMI, kg/m2
|
22.75±3.16 |
23.10±4.52 |
0.542 |
|
Smoking |
75 (47.8) |
41 (37.6) |
0.100 |
|
Alcohol |
65 (41.4) |
32 (29.4) |
0.045 |
|
Creatinine, mg/dL |
2.38±7.09 |
1.69±1.53 |
0.163 |
|
Albumin, mg/dL |
3.47±0.79 |
3.36±0.82 |
0.234 |
|
Hemoglobin, mg/dL |
11.70±2.07 |
11.03±1.73 |
0.002 |
|
WBC, K/μL |
11.08±6.33 |
8.96±4.16 |
<0.001 |
|
ESR, mm/hr |
62.95±42.11 |
56.33±36.22 |
0.159 |
|
CRP, mg/dL |
6.91±9.44 |
3.91±6.39 |
0.003 |
|
Total cholesterol, mg/dL |
169.25±63.95 |
159.35±48.12 |
0.178 |
|
Triglyceride, mg/dL |
162.52±188.03 |
151.29±111.90 |
0.602 |
|
HDL-C, mg/dL |
39.59±16.52 |
34.27±14.98 |
0.010 |
|
LDL-C, mg/dL |
97.61±56.70 |
85.69±51.34 |
0.061 |
|
Nephropathy |
147 (64.2) |
62 (56.9) |
0.150 |
|
Retinopathy |
101 (63.1) |
39 (37.1) |
<0.001 |
|
Neuropathy |
118 (74.2) |
45 (42.9) |
<0.001 |
|
Coronary artery disease |
29 (18.0) |
18 (17.1) |
0.856 |
|
Cerebrovascular disease |
29 (18.2) |
19 (18.1) |
0.976 |
|
Peripheral arterial disease |
29 (18.7) |
31 (29.5) |
0.042 |
|
Oral hypoglycemic agent |
155 (71.0) |
86 (66.7) |
0.401 |
|
Insulin |
23 (62.2) |
23 (73.3) |
0.034 |
|
Statin |
59 (27.0) |
45 (35.2) |
0.107 |
|
Aspirin |
81 (37.1) |
51 (40.0) |
0.591 |
|
Clopidogrel |
41 (18.9) |
30 (23.8) |
0.277 |
|
Warfarin |
8 (3.77) |
8 (6.67) |
0.224 |

In terms of microvascular complications, the proportion of diabetic nephropathy was similar (64.2% vs. 56.6%, P=0.15), while the proportions of diabetic retinopathy (63.1% vs. 37.1%, P<0.001) and diabetic neuropathy (74.2% vs. 42.9%, P<0.001) were reduced in group B. In terms of macrovascular complications, neither the proportion of CAD (18.0% vs. 17.1%, P=0.856) nor that of cerebrovascular disease (18.2% vs. 18.1%, P=0.976) differed significantly between the two groups, while an increase in the proportion of peripheral vascular disease (18.7% vs. 29.5%, P=0.042) was noted in group B.
When medication was compared, no significant difference in the use of oral hypoglycemic agents (71.0% vs. 66.7%, P= 0.401), statins (27.0% vs. 35.2%, P=0.107), aspirin (37.1% vs. 40.0%, P=0.591), clopidogrel (18.9% vs. 23.8%, P=0.277), or warfarin (3.77% vs. 6.67%, P=0.224) was noted, while an increase in the use of insulin (62.2% vs. 73.3%, P=0.034) was noted in group B. Despite a decrease in alcohol consumption (41.4% vs. 29.4%, P=0.045) in group B, smoking was similar (47.8% vs. 37.6%, P=0.100).
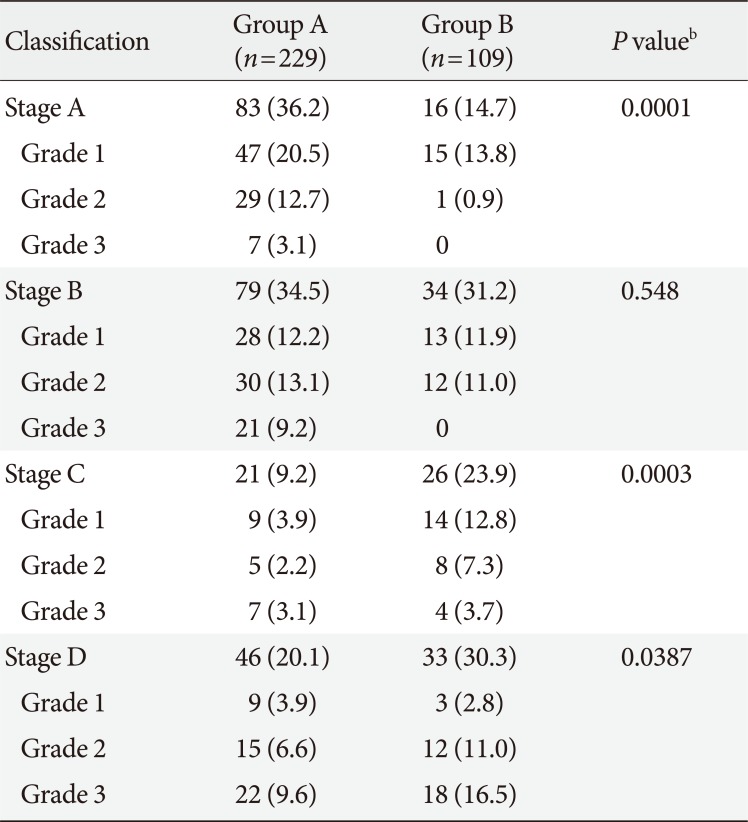
In terms of DFU severity based on Wagner classification system, the depth and degree of DFU were similar between the two groups (
Supplementary Table 1). However, a noticeable change was observed when using the UT classification system. The proportion of stage B ulcers, or ulcers complicated by infection, was similar between groups (34.5% to 31.2%,
P=0.548), while the proportion of stage C ulcers, or ulcers complicated by vascular ischemia, increased from 9.4% to 28.7% (
P=0.0003). In addition, the proportion of stage D ulcers which complicated by both vascular ischemia and infection increased from 20.1% to 30.2% (
P=0.0387) (
Table 2).
Table 2
Degree of diabetic foot ulcer on admission according to University of Texas classification in group A and group Ba
|
Classification |
Group A (n=229) |
Group B (n=109) |
P valueb
|
|
Stage A |
83 (36.2) |
16 (14.7) |
0.0001 |
|
Grade 1 |
47 (20.5) |
15 (13.8) |
|
Grade 2 |
29 (12.7) |
1 (0.9) |
|
Grade 3 |
7 (3.1) |
0 |
|
Stage B |
79 (34.5) |
34 (31.2) |
0.548 |
|
Grade 1 |
28 (12.2) |
13 (11.9) |
|
Grade 2 |
30 (13.1) |
12 (11.0) |
|
Grade 3 |
21 (9.2) |
0 |
|
Stage C |
21 (9.2) |
26 (23.9) |
0.0003 |
|
Grade 1 |
9 (3.9) |
14 (12.8) |
|
Grade 2 |
5 (2.2) |
8 (7.3) |
|
Grade 3 |
7 (3.1) |
4 (3.7) |
|
Stage D |
46 (20.1) |
33 (30.3) |
0.0387 |
|
Grade 1 |
9 (3.9) |
3 (2.8) |
|
Grade 2 |
15 (6.6) |
12 (11.0) |
|
Grade 3 |
22 (9.6) |
18 (16.5) |

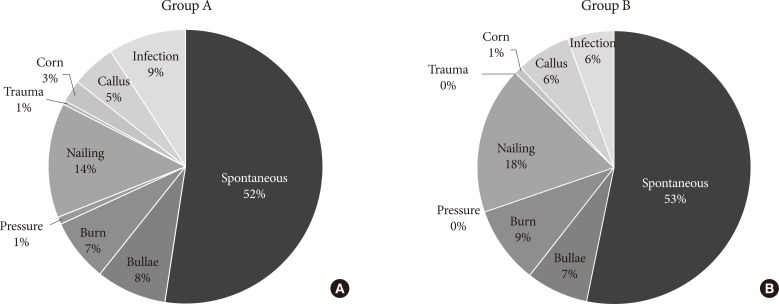
The reported cause of DFU for both groups was summarized in
Fig. 1. Spontaneous development was the leading reported cause of DFU in both groups, followed by traumatic cause. The proportion of infection, the third leading reported cause of diabetic foot in group A, decreased from 9% to 6% (
P=0.19).
Fig. 1
Reported causes of diabetic foot ulcer in (A) group A and (B) group B.

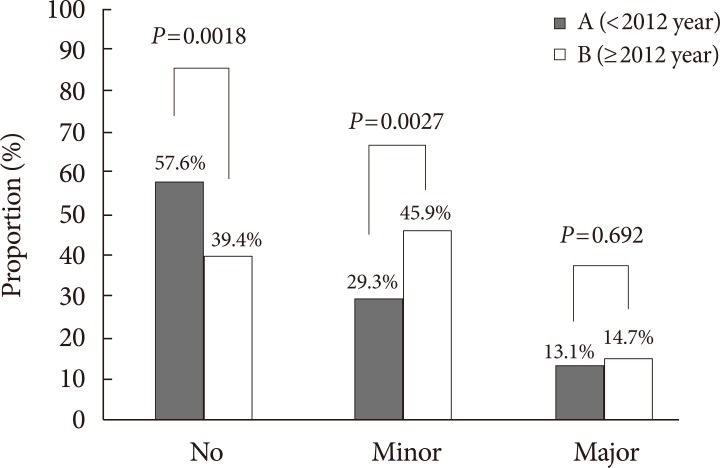
Out of 229 patients in group A, 97 cases of LEA (about 9.7 amputation cases/year) was documented, while out of 109 patients in group B, 68 cases of LEA (about 17 amputation cases/ year) was documented. Of these cases, 27 cases in group A (27.84%), and 20 cases in group B (29.41%) were multiple amputations (
P=0.825). Comparison of amputation rates for both groups is summarized in
Fig. 2. Conservative treatment, or no amputation, was the predominant method of diabetic foot treatment in group A, while minor LEA was the most performed method of treatment for group B. The proportion of conservative treatment decreased (57.64% vs. 39.44%,
P=0.0018), while the proportion of minor LEA increased (29.26% vs. 45.87%,
P=0.0027). The proportion of major LEA remained similar (13.10% vs. 14.67%,
P=0.692) (
Fig. 2). When minor LEA was subclassified into forefoot and mid/hindfoot amputations, a significant increase in forefoot amputation was noted (19.65% vs. 32.11%,
P=0.012), while the same significant increase was not observed for mid/hindfoot amputation (9.17% vs. 15.60%,
P=0.0807). When major LEA was subclassified into below knee amputations and above knee amputations, no significant difference was noted for both (11.79% vs. 11.01%,
P=0.8341; 1.75% vs. 3.67%,
P=0.2787, respectively).
Fig. 2
Comparison of the proportion of lower extremity amputations between group A and group B. No, no amputation; Minor, below-ankle amputation; Major, above-ankle amputation.

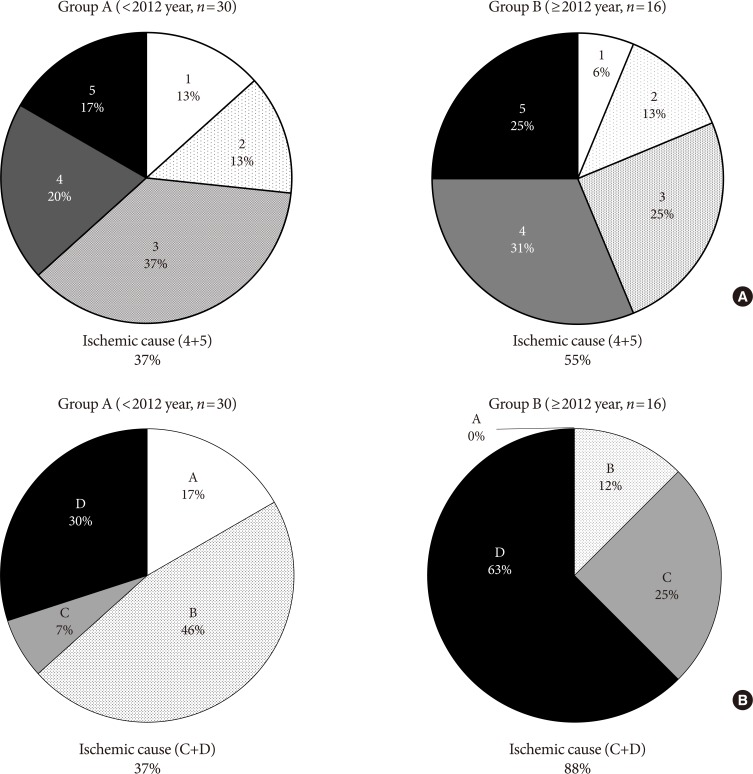
Although the rate of major amputation remained similar, etiology of major amputation were changed when compared between group A and B by two different classification system (Wagner and UT). Critical ischemic limb had been identified as the main cause of major amputation from analysis of both classification (
Fig. 3).
Fig. 3
Comparison of major amputation causes according to (A) Wagner classification system and (B) University of Texas classification between groups.

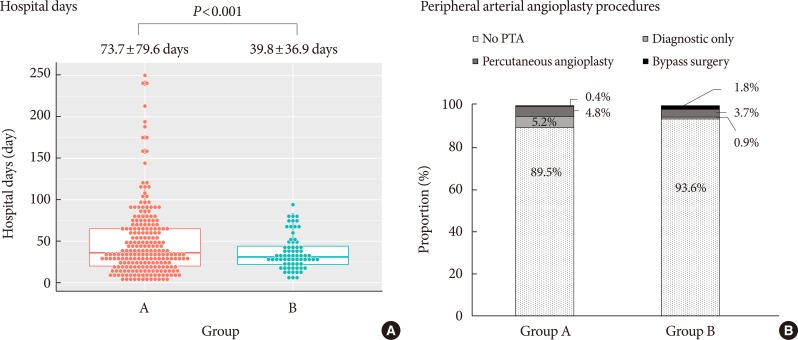
There was a significant decrease in the mean length of hospital stay, from 73.7 to 39.8 days (
P<0.001) (
Fig. 4A). Comparison of peripheral angiography procedures was summarized in
Fig. 4B. Twenty-six patients in group A (11.35%), while 37 patients in group B (33.94%) underwent peripheral angiography. The proportion of cases not undergoing angiography decreased significantly from 84.88% to 71.09% (
P=0.004). While the proportion of diagnostic-only peripheral angiography procedures was not different (11.63% vs. 7.81%,
P=0.2773), the proportion of peripheral angiography with balloon angioplasty (1.74% vs. 12.5%,
P=0.0002) or stent insertion (1.74% vs. 8.59%,
P=0.0055) increased significantly.
Fig. 4
Comparison of (A) length of admission and (B) peripheral percutaneous transluminal angiography (PTA) procedures between group A and group B.

DISCUSSION
In this study, we identified the changes of characteristics over the past 10 years and the trends in treatment outcomes in patients with DFU. Patients in group B (admitted after 2012) had better glycemic control in terms of reduced HbA1c, less diabetic microvascular complications, and reduced length of hospital stay despite longer duration of diabetes. A decrease in the proportion of infected cause of DFU and an increase in ischemic cause of DFU were observed. Although the proportion of minor LEA was increased, the proportions of major LEAs did not change significantly.
In many previous studies, LEA outcome was used as the primary clinical endpoint of DFU treatment. A retrospective study involving a single center in Copenhagen from 1981 to 1995 found a 75% reduction in the incidence of major leg amputations following the introduction of a multidisciplinary diabetic foot clinic and a 7-fold increase in revascularization procedures [
9]. A similar single-center study involving 11,332 patients with diabetes in Denmark also found a significant reduction in the incidence of major LEAs [
8]. A significant reduction in the incidence of total and major LEA (30% and 41%, respectively) was found in a retrospective cohort study of the Scottish morbidity records [
15]. In a prospective study at a single center in the United Kingdom during an 11-year period, the incidence of major LEAs fell by 62% when improvements to foot care were made through multidisciplinary team work and continuous prospective audit [
10]. While the incidence of major LEAs was significantly reduced in these studies, this study did not find a significant reduction of major LEAs in terms of change in proportion.
Several factors may have contributed to the LEA outcome in our study. We hypothesize that improved glycemic control and increased duration of diabetes seen in group B, together with an increase in incidence of ischemic cause of DFU determined by UT classification system suggests that prolonged exposure to hyperglycemia might have adversely altered the peripheral arterial vasculature which, despite improved glycemic control, resulted in more cases requiring minor LEAs. Increased duration of diabetes (or delayed onset) might have adversely affected the peripheral arterial vasculature of DFU patients through prolonged exposure to atherosclerosis, arterial stiffness, and hyperglycemia-induced microvascular dysfunction [
1617], leading to increased proportion of peripheral vascular disease. A study on American Pima Indians [
6], as well as in a study in Finland [
7] have found that peripheral arterial disease is associated with LEA in patients with DFU. In Holstein et al. [
9], a 75% reduction in the incidence of major leg amputations following the introduction of a multidisciplinary diabetic foot clinic and a 7-fold increase in revascularization procedures was achieved, in part, due to a 7-fold increase in revascularization procedures. In this study, although an increase in the proportion of peripheral transluminal angioplasty was observed, the small population size may not have had enough statistical power to have caused an impact on LEA outcomes. In addition, the similar proportion of major LEA in our study may have simply reflected the steady-state level of DFU patients with critical limb ischemia of the local region. Overall, determining the causality of increased peripheral vascular disease as a contributing factor for increased LEA is limited mainly by the retrospective cross-sectional design of this study.
Although this study used LEA as the primary endpoint of DFU outcome, this notion has been challenged by some authors. The decision to use the ankle level as the anatomic basis for the severity of LEA has been criticized in some studies due to the fact that this level does not directly correlate with functional impairment [
18]. In Quigley et al. [
19], a systematic review of quality of life in patients with either a partial foot amputation and transtibial amputation have found that quality of life of both groups were similar. In addition, patients with LEA above the ankle may still be able to preserve significant functional capacity through adequate rehabilitation programs and use of prosthesis. In Gauthier-Gagnon et al. [
20], more than 90% of patients with transtibial and transfemoral amputations were able to perform basic activities like walking in the house or standing from a chair. Although most studies on diabetes-related LEA in the current literature used a dichotomous classification of LEA severity with the ankle level as the reference point of severity [
81015], some studies have classified LEA based on the anatomical level according to the surgeon's point of view. In Gurlek et al. [
21], LEA was classified into transphalangeal, transmetatarsal Syme type, below knee, and above knee amputations. One strength of this study is in classifying LEA based on both the level of the ankle and on the anatomical level according to surgeon's point of view.
In this study, an increase in minor LEA following multidisciplinary approach was observed. A similar result was found in a study Holstein et al. [
9]. When we tried to subdivided the minor LEA group based on a more specified anatomic level, we found that the increase in minor LEA proportion was mostly due to an increase of digital/ray amputations or amputations involving a single digit (data not shown). We believe that the increase of minor LEA does not necessarily reflect a failure of adequate DFU management, but as part of a strategy for limbsalvage. Many studies that investigated the role of limb salvage in DFU, defined as LEA below the ankle level resulting in the preservation of the ankle, have found that adequate and timely limb salvage resulting in ankle preservation resulted in improved mortality and morbidity, as well as favorable postoperative functional outcome [
182223]. The multidisciplinary approach helped clinicians reach decisions regarding timing of amputation earlier, resulting in reduced length of hospital stay, which was observed in this study.
As the two most commonly used DFU classification systems, Wagner classification system and UT classification system were used to assess the severity of DFUs in this study. The different outcomes between the two different classification systems may have arisen due to the increased descriptive power by the UT classification system, which in addition to depth of ulcer, provides information regarding the presence of infection or clinical signs of lower extremity ischemia (
Table 2,
Fig. 3) [
14]. According to Wagner classification, ischemic foot ulcer without gangrene (even in localized)—i.e., bluish digit or ulcer with ischemic pain—is not classified as grade 4 or 5. Although the rate of major amputation remains similar, an increase in ischemic causes in both classification systems is considered to be an important implication of this study and efforts should be made to detect early ischemic changes in clinical practice (
Fig. 3).
In Korea, epidemiologic studies on DFU have been mostly single-center based, retrospective, and either limited or a part of a broader prevalence study of general diabetes-related complications. A study on DFU patients, based on the National Health Insurance Database from years 1994 to 2002, found that the diabetic population suffered from a nearly 10 times higher prevalence of foot ulcer disease and LEAs, nearly twice as high total medical costs, and longer length of hospital stay when compared with the data from nondiabetic population [
24]. A 5-year observational study of 508 patients with DFU from a tertiary referral center showed that both diabetic retinopathy (odds ratio [OR], 6.707) and autonomic neuropathy (OR, 3.967) were factors positively associated with the development of DFU, while HbA1c was an independent risk factor of DFU, which suggested the importance of glycemic control and routine examinations for diabetes-related complications [
25]. A study from another single tertiary referral center of 72 patients with DFU demonstrated that infection was the leading cause of DFU and admission within 3 days of onset of symptoms was a statistically significant factor in reducing major amputations and repeated debridement [
26]. Compared with these studies, our study was unique in that every DFU case was assessed by an endocrinologist, and DFU severity was graded based on predefined staging systems. Also, to our knowledge, this study was the first in Korea to describe treatment outcomes of DFU patients following the introduction of a multidisciplinary approach.
This study had a number of limitations. As mentioned earlier, due to the retrospective cross-sectional study design, causality between exposure and outcome cannot be definitely established. The role of peripheral vascular disease in the treatment of DFU, as well as the potential benefit of treating peripheral vascular disease through vascular intervention in terms of reducing LEA outcomes, remains to be elucidated. Based on selective retrospective review of medical records of patients who were eligible candidates for percutaneous transluminal angiography in terms of low ABI, we found that not all eligible candidates ended up performing the procedure. In most instances, medical records documented refusal on the patient's side due to a variety of reasons, including prohibitive costs, comorbidities such as old age, and preference for nonsurgical conservative treatment. Due to the single center design, the study population may not have accurately reflected the loco-regional population. Yet, the issue of deriving a valid regional incidence rate was also challenged in other studies by the difficulty in obtaining a valid estimate of the regional diabetic population, regional diabetic population at risk of developing LEA, and number of LEA cases performed within the region [
8]. In addition, since tertiary referral centers not only have a substantial and steady number of outpatient T2DM patients with or at risk of DFU, but also accept a broad range of complicated DFU cases from the region in potential need of surgical treatment, the results observed in this study may have nonetheless provided some insight on the overall trend of the region. This study did not investigate other secondary outcomes resulting from complications of DFU, such as the difference in cultured pathogens, use of antibiotics, development of sepsis, and mortality. In addition, the study did account for the actual financial cost or the posttreatment functional outcome of the patient. Whether the increase in minor LEAs and decrease in length of hospital stay translate into reduced financial burden is uncertain.
The strength of this study lies in its reproducibility, assessment of anthropomorphic and biochemical markers of glycemic control, comparison of incidence of diabetic microvascular and macrovascular complications, classification of every DFU based on predefined staging system, and comparison of surgical treatment outcomes and length of hospital stay. A prospective, nationwide cohort study with an accurate ascertainment of all regional patients with diabetes, with an emphasis on the impact of peripheral vascular disease and vascular intervention outcomes on the incidence of major LEA could amend many aforementioned shortcomings.
In conclusion, over the course of 10 years, the diabetic foot population at a single tertiary referral center in Korea has experienced better glycemic control, less diabetic microvascular complications, less infectious cause of DFU, more ischemic cause of DFU, and longer duration of diabetes. The introduction of multidisciplinary treatment resulted in an increase in minor LEAs and reduced length of hospital stay, which may have resulted in positive health and economic benefits to the diabetic foot population. Improved overall glycemic control and reduced diabetic microvascular complications did translate into overall improved DFU severity or decrease in major LEAs. The results of this study suggest the need for further prospective cohort study on the prevalence of peripheral vascular disease and its causality in relations to LEA in the DFU population, the impact of revascularization in LEA outcomes, and the use of clinical outcomes such as post-treatment functional capacity as clinical endpoints in the DFU population.






 PDF
PDF ePub
ePub Citation
Citation Print
Print







 XML Download
XML Download