Abstract
Background
Methods
Results
ACKNOWLEDGMENTS
References
Fig. 1
Serotonin inhibition protected against high fat diet (HFD)-induced hepatic steatosis. Eight-week-old mice were fed a standard chow diet (SCD) or HFD for 12 weeks with vehicle, para-chlorophenylalanine (PCPA), or LP-533401 treatment. (A) H&E staining of liver sections from SCD- or HFD-fed mice with vehicle or PCPA treatment. (B) Quantification of hepatic triglyceride (TG) levels in PCPA-treated mice (n=6). (C–E) H&E staining of liver sections (left) and quantification of hepatic TG levels (right) from HFD-fed mice treated with LP-533401 (C), fat-specific Tph1-knockout (Tph1 FKO) mice (D), and 5-hydroxytryptamine receptor 3A (Htr3a) knockout (KO) mice (E) (n=6). Representative images are shown. Scale bars, 50 µm. Tphfl/fl, tryptophan hydroxylase 1 floxed. aP<0.05, bP<0.01, cP<0.001.
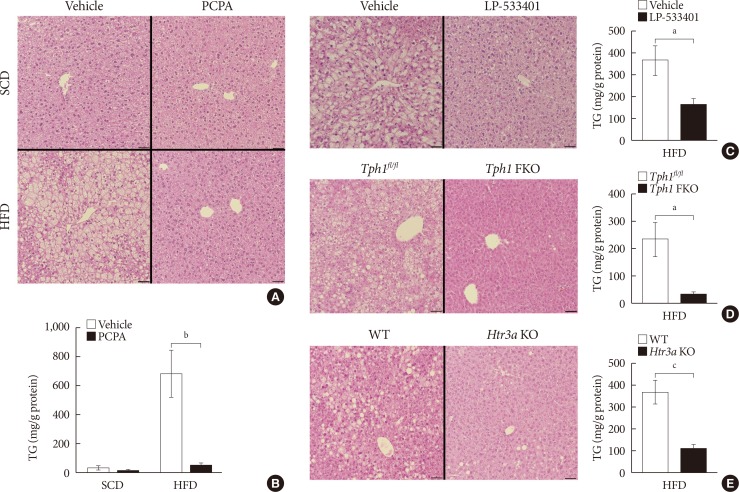
Fig. 2
Para-chlorophenylalanine (PCPA) treatment suppressed the positive hepatic lipid balance. Eight-week-old mice were fed a standard chow diet (SCD) or high fat diet (HFD) for 12 weeks and treated with vehicle or PCPA treatment. Hepatic expressional profiles of genes related to de novo lipogenesis (A), triglyceride synthesis (B), fatty acid (FA) uptake (C), FA oxidation (D), very low density lipoprotein secretion (E), and transcription factors (F) were assessed by quantitative reverse transcription polymerase chain reaction (n=6). Acaca, acetyl-CoA carboxylase alpha; Fasn, fatty acid synthase; Acly, ATP citrate lyase; Me1, malic enzyme 1; Scd1, stearoyl-CoA desaturase 1; Gpam, glycerol-3-phosphate acyltransferase; Agpat1, 1-acylglycerol-3-phosphate O-acyltransferase 1; Lpin1, lipin 1; Mogat1, monoacylglycerol O-acyltransferase 1; Dgat1, diacylglycerol O-acyltransferase 1; Dgat2, diacylglycerol O-acyltransferase 2; Cpt1a, carnitine palmitoyltransferase 1a; Mttp, microsomal triglyceride transfer protein; Apob, apolipoprotein B; Pparg, peroxisome proliferator activated receptor gamma; Ppargc1a, Pparg coactivator 1 alpha; Srebp1c, sterol regulatory element binding transcription factor 1c; Mlxipl, MLX interacting protein-like (ChREBP, carbohydrate response element binding protein); Nr1h3, nuclear receptor subfamily 1, group H, member 3 (LXR, liver X receptor). aP<0.05, bP<0.01, cP<0.001.
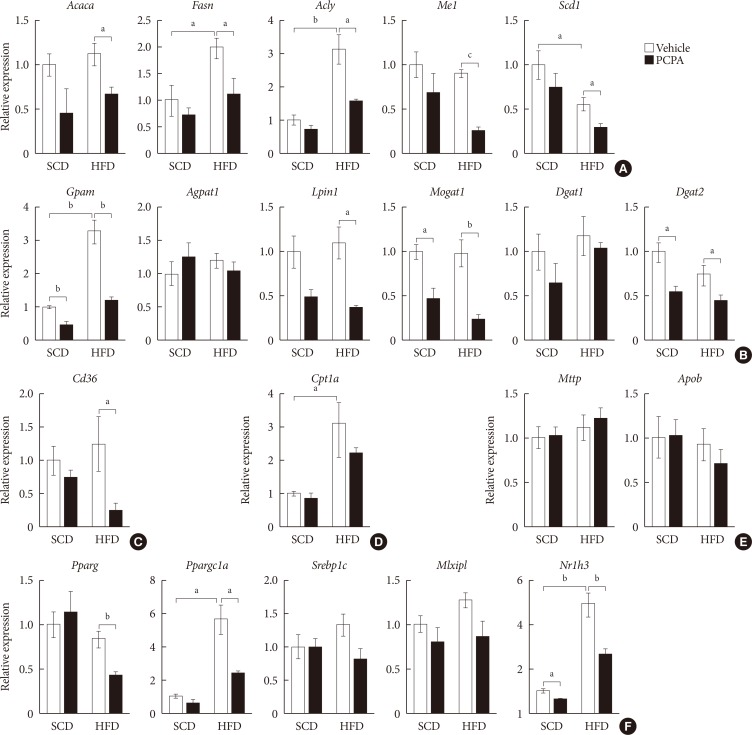
Fig. 3
Short-term treatment with para-chlorophenylalanine (PCPA) in the context of high carbohydrate diet (HCD) protected against hepatic steatosis independently from energy expenditure and insulin sensitivity. Twelve-week-old mice were fed a standard chow diet (SCD) or HCD for 2 weeks and treated with vehicle or PCPA treatment. Intraperitoneal glucose tolerance tests (A) and insulin tolerance tests (B) were performed (n=4). (C) H&E staining of inguinal white adipose tissue sections from HCD-fed mice with vehicle or PCPA treatment. (D) H&E staining of liver sections from SCD- or HCD-fed mice with vehicle or PCPA treatment. (E) Quantification of hepatic triglyceride levels in PCPA-treated mice (n=6). Representative images are shown. Scale bars, 50 µm. aP<0.05.
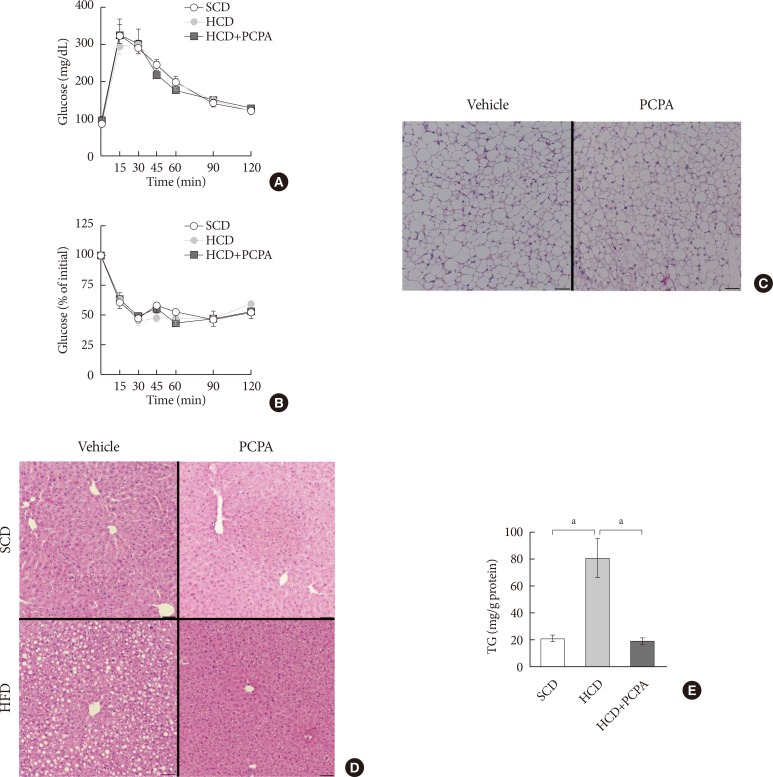
Fig. 4
Para-chlorophenylalanine (PCPA) treatment suppressed the positive hepatic lipid balance via downregulation of Pparg, Srebp1c, and Mlxipl. Twelve-week-old mice were fed a standard chow diet (SCD) or high carbohydrate diet (HCD) for 2 weeks with vehicle or PCPA treatment. Hepatic expressional profiles of genes related to de novo lipogenesis (A), triglyceride synthesis (B), fatty acid (FA) uptake (C), FA oxidation (D), very low density lipoprotein secretion (E), and transcription factors (F) were assessed by quantitative reverse transcription polymerase chain reaction (n=6). Acaca, acetyl-CoA carboxylase alpha; Fasn, fatty acid synthase; Acly, ATP citrate lyase; Me1, malic enzyme 1; Scd1, stearoyl-CoA desaturase 1; Gpam, glycerol-3-phosphate acyltransferase; Agpat1, 1-acylglycerol-3-phosphate O-acyltransferase 1; Lpin1, lipin 1; Mogat1, monoacylglycerol O-acyltransferase 1; Dgat1, diacylglycerol O-acyltransferase 1; Dgat2, diacylglycerol O-acyltransferase 2; Cpt1a, carnitine palmitoyltransferase 1a; Apob, apolipoprotein B; Mttp, microsomal triglyceride transfer protein; Pparg, peroxisome proliferator activated receptor gamma; Ppargc1a, Pparg coactivator 1 alpha; Srebp1c, sterol regulatory element binding transcription factor 1c; Mlxipl, MLX interacting protein-like (ChREBP, carbohydrate response element binding protein); Nr1h3, nuclear receptor subfamily 1, group H, member 3 (LXR, liver X receptor). aP<0.05, bP<0.001.
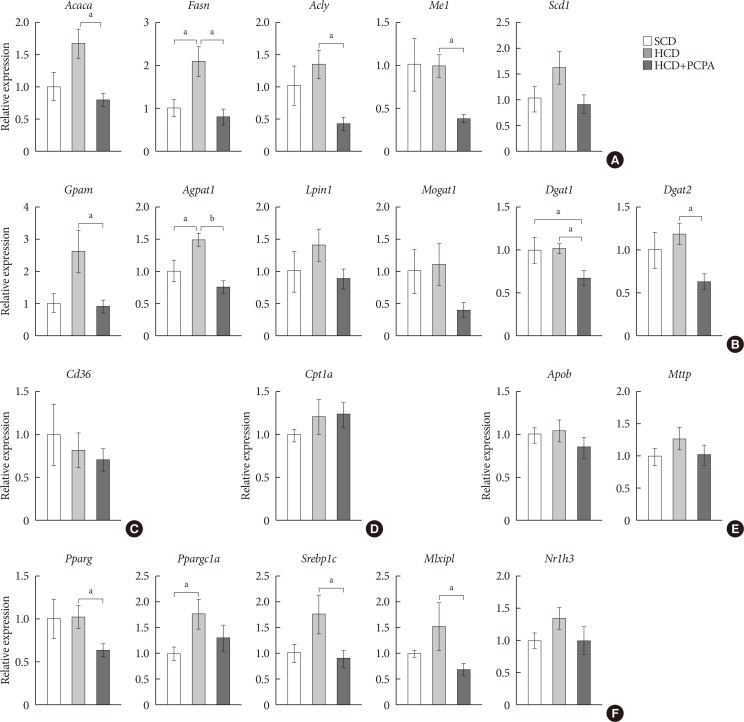
Table 1
Sequences of primers used in mouse genotyping
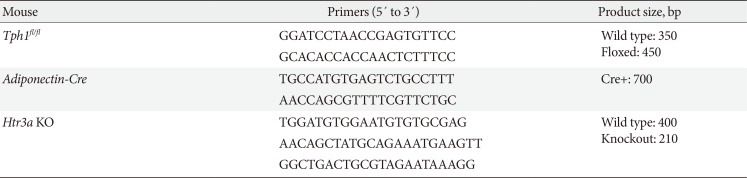
Table 2
Sequences of primers used in quantitative real-time polymerase chain reaction
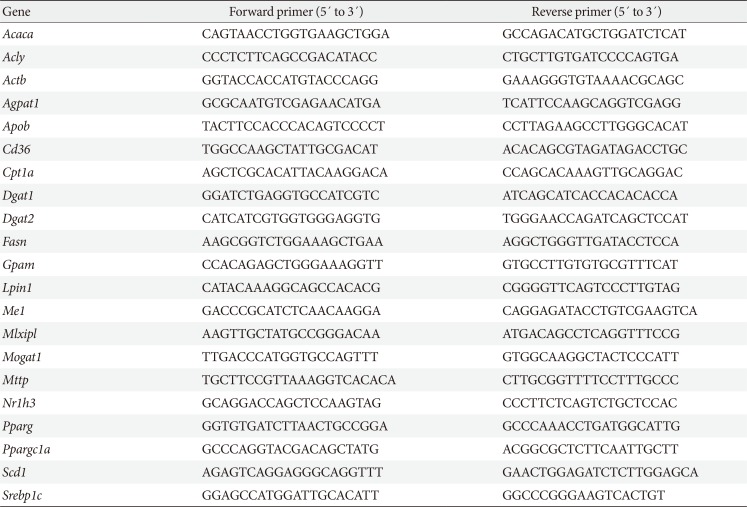
Acaca, acetyl-CoA carboxylase alpha; Acly, ATP citrate lyase; Actb, actin beta; Agpat1, 1-acylglycerol-3-phosphate O-acyltransferase 1; Apob, apolipoprotein B; Cpt1a, carnitine palmitoyltransferase 1a; Dgat1, diacylglycerol O-acyltransferase 1; Dgat2, diacylglycerol O-acyltransferase 2; Fasn, fatty acid synthase; Gpam, glycerol-3-phosphate acyltransferase; Lpin1, lipin 1; Me1, malic enzyme 1; Mlxipl, MLX interacting protein-like (ChREBP, carbohydrate response element binding protein); Mogat1, monoacylglycerol O-acyltransferase 1; Mttp, microsomal triglyceride transfer protein; Nr1h3, nuclear receptor subfamily 1, group H, member 3 (LXR, liver X receptor); Pparg, peroxisome proliferator activated receptor gamma; Ppargc1a, Pparg coactivator 1 alpha; Scd1, stearoyl-CoA desaturase 1; Srebp1c, sterol regulatory element binding transcription factor 1c.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download