1. Genuth S, Alberti KG, Bennett P, Buse J, Defronzo R, Kahn R, Kitzmiller J, Knowler WC, Lebovitz H, Lernmark A, Nathan D, Palmer J, Rizza R, Saudek C, Shaw J, Steffes M, Stern M, Tuomilehto J, Zimmet P. Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003; 26:3160–3167. PMID:
14578255.
2. Tai ES, Goh SY, Lee JJ, Wong MS, Heng D, Hughes K, Chew SK, Cutter J, Chew W, Gu K, Chia KS, Tan CE. Lowering the criterion for impaired fasting glucose: impact on disease prevalence and associated risk of diabetes and ischemic heart disease. Diabetes Care. 2004; 27:1728–1734. PMID:
15220254.
3. Tirosh A, Shai I, Tekes-Manova D, Israeli E, Pereg D, Shochat T, Kochba I, Rudich A. Israeli Diabetes Research Group. Normal fasting plasma glucose levels and type 2 diabetes in young men. N Engl J Med. 2005; 353:1454–1462. PMID:
16207847.

4. Nichols GA, Hillier TA, Brown JB. Normal fasting plasma glucose and risk of type 2 diabetes diagnosis. Am J Med. 2008; 121:519–524. PMID:
18501234.

5. Brambilla P, La Valle E, Falbo R, Limonta G, Signorini S, Cappellini F, Mocarelli P. Normal fasting plasma glucose and risk of type 2 diabetes. Diabetes Care. 2011; 34:1372–1374. PMID:
21498787.

6. Meigs JB, Muller DC, Nathan DM, Blake DR, Andres R. Baltimore Longitudinal Study of Aging. The natural history of progression from normal glucose tolerance to type 2 diabetes in the Baltimore Longitudinal Study of Aging. Diabetes. 2003; 52:1475–1484. PMID:
12765960.

7. Qiao Q, Hu G, Tuomilehto J, Nakagami T, Balkau B, Borch-Johnsen K, Ramachandran A, Mohan V, Iyer SR, Tominaga M, Kiyohara Y, Kato I, Okubo K, Nagai M, Shibazaki S, Yang Z, Tong Z, Fan Q, Wang B, Chew SK, Tan BY, Heng D, Emmanuel S, Tajima N, Iwamoto Y, Snehalatha C, Vijay V, Kapur A, Dong Y, Nan H, Gao W, Shi H, Fu F. DECODA Study Group. Age- and sex-specific prevalence of diabetes and impaired glucose regulation in 11 Asian cohorts. Diabetes Care. 2003; 26:1770–1780. PMID:
12766108.
8. DECODE Study Group. Age- and sex-specific prevalences of diabetes and impaired glucose regulation in 13 European cohorts. Diabetes Care. 2003; 26:61–69. PMID:
12502659.
9. Yang W, Lu J, Weng J, Jia W, Ji L, Xiao J, Shan Z, Liu J, Tian H, Ji Q, Zhu D, Ge J, Lin L, Chen L, Guo X, Zhao Z, Li Q, Zhou Z, Shan G, He J. China National Diabetes and Metabolic Disorders Study Group. Prevalence of diabetes among men and women in China. N Engl J Med. 2010; 362:1090–1101. PMID:
20335585.

10. Araneta MR, Kanaya AM, Hsu WC, Chang HK, Grandinetti A, Boyko EJ, Hayashi T, Kahn SE, Leonetti DL, McNeely MJ, Onishi Y, Sato KK, Fujimoto WY. Optimum BMI cut points to screen asian americans for type 2 diabetes. Diabetes Care. 2015; 38:814–820. PMID:
25665815.

11. Fujimoto WY, Leonetti DL, Kinyoun JL, Shuman WP, Stolov WC, Wahl PW. Prevalence of complications among second-generation Japanese-American men with diabetes, impaired glucose tolerance, or normal glucose tolerance. Diabetes. 1987; 36:730–739. PMID:
3569672.

12. Koppes LL, Dekker JM, Hendriks HF, Bouter LM, Heine RJ. Moderate alcohol consumption lowers the risk of type 2 diabetes: a meta-analysis of prospective observational studies. Diabetes Care. 2005; 28:719–725. PMID:
15735217.
13. Paffenbarger RS Jr, Wing AL, Hyde RT. Physical activity as an index of heart attack risk in college alumni. Am J Epidemiol. 1978; 108:161–175. PMID:
707484.

14. Bergstrom RW, Leonetti DL, Newell-Morris LL, Shuman WP, Wahl PW, Fujimoto WY. Association of plasma triglyceride and C-peptide with coronary heart disease in Japanese-American men with a high prevalence of glucose intolerance. Diabetologia. 1990; 33:489–496. PMID:
2210122.

15. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985; 28:412–419. PMID:
3899825.
16. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 1997; 20:1183–1197. PMID:
9203460.
17. Yoon KH, Lee JH, Kim JW, Cho JH, Choi YH, Ko SH, Zimmet P, Son HY. Epidemic obesity and type 2 diabetes in Asia. Lancet. 2006; 368:1681–1688. PMID:
17098087.

18. Kahn SE. The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of type 2 diabetes. Diabetologia. 2003; 46:3–19. PMID:
12637977.

19. Jensen CC, Cnop M, Hull RL, Fujimoto WY, Kahn SE. American Diabetes Association GENNID Study Group. Beta-cell function is a major contributor to oral glucose tolerance in high-risk relatives of four ethnic groups in the U.S. Diabetes. 2002; 51:2170–2178. PMID:
12086947.
20. Fukushima M, Suzuki H, Seino Y. Insulin secretion capacity in the development from normal glucose tolerance to type 2 diabetes. Diabetes Res Clin Pract. 2004; 66(Suppl 1):S37–S43. PMID:
15563978.

21. Fujimoto WY, Boyko EJ, Hayashi T, Kahn SE, Leonetti DL, McNeely MJ, Shuman WP. Risk factors for type 2 diabetes: lessons learned from Japanese Americans in Seattle. J Diabetes Investig. 2012; 3:212–224.

22. DECODA Study Group. International Diabetes Epidemiology Group. Cardiovascular risk profile assessment in glucose-intolerant Asian individuals: an evaluation of the World Health Organization two-step strategy. The DECODA Study (Diabetes Epidemiology: Collaborative Analysis of Diagnostic Criteria in Asia). Diabet Med. 2002; 19:549–557. PMID:
12099957.
23. DECODE Study Group on behalf of the European Diabetes Epidemiology Study Group. Will new diagnostic criteria for diabetes mellitus change phenotype of patients with diabetes? Reanalysis of European epidemiological data. BMJ. 1998; 317:371–375. PMID:
9694750.
24. Suzuki H, Fukushima M, Usami M, Ikeda M, Taniguchi A, Nakai Y, Matsuura T, Kuroe A, Yasuda K, Kurose T, Seino Y, Yamada Y. Factors responsible for development from normal glucose tolerance to isolated postchallenge hyperglycemia. Diabetes Care. 2003; 26:1211–1215. PMID:
12663599.

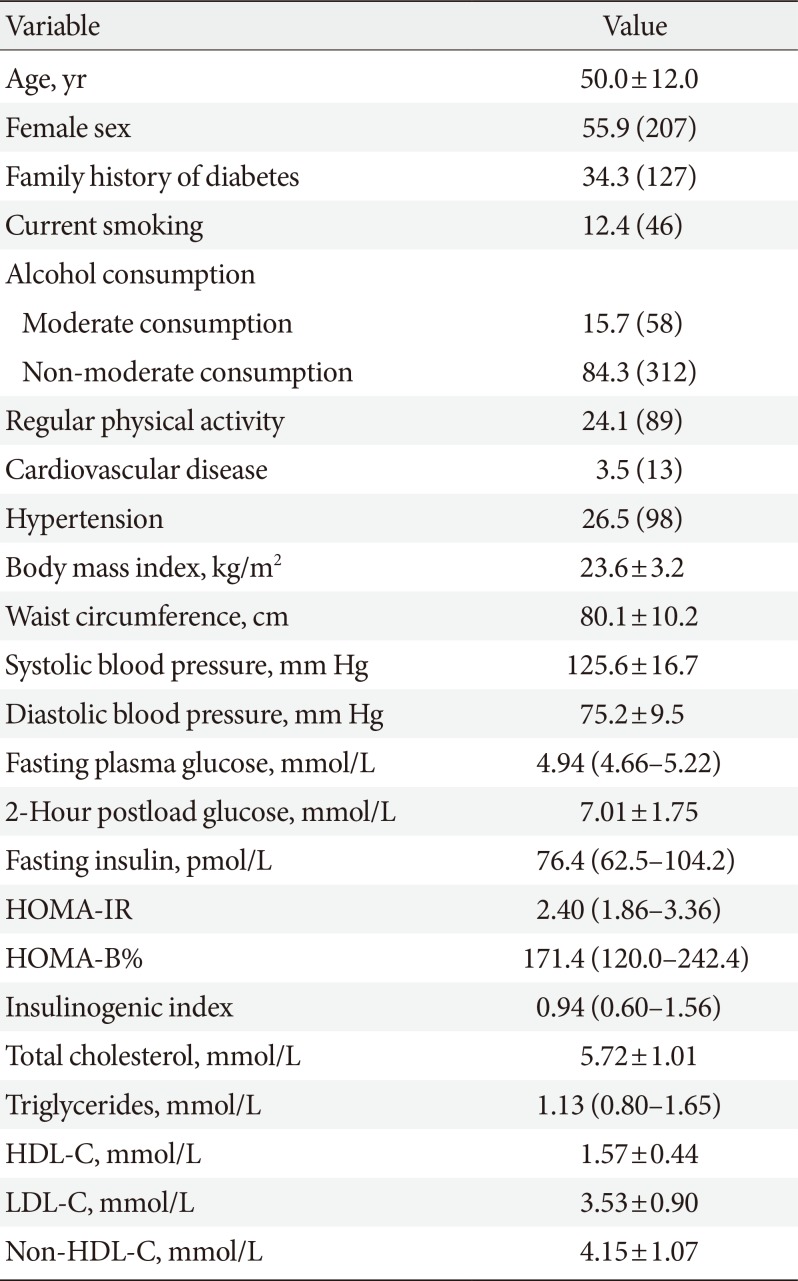
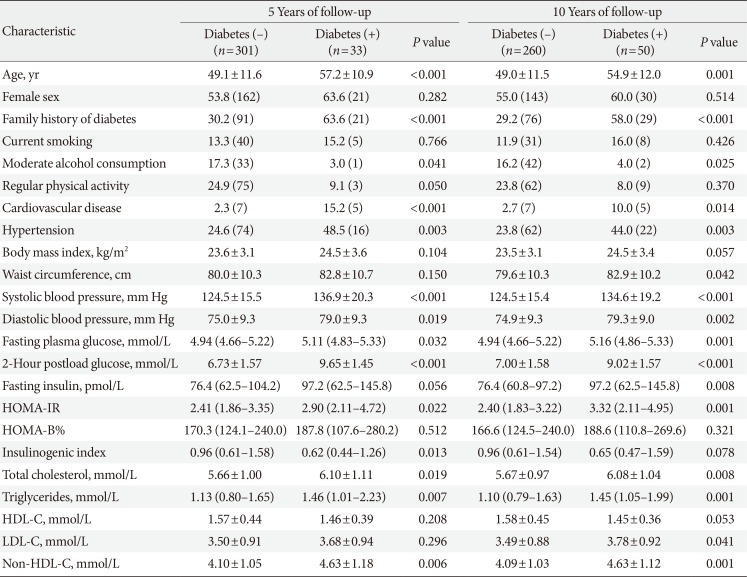
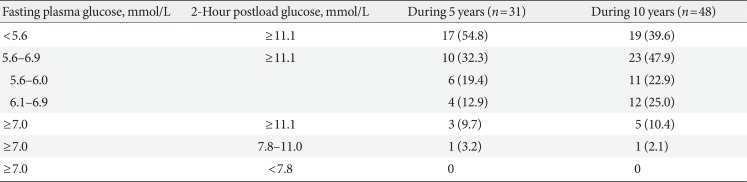
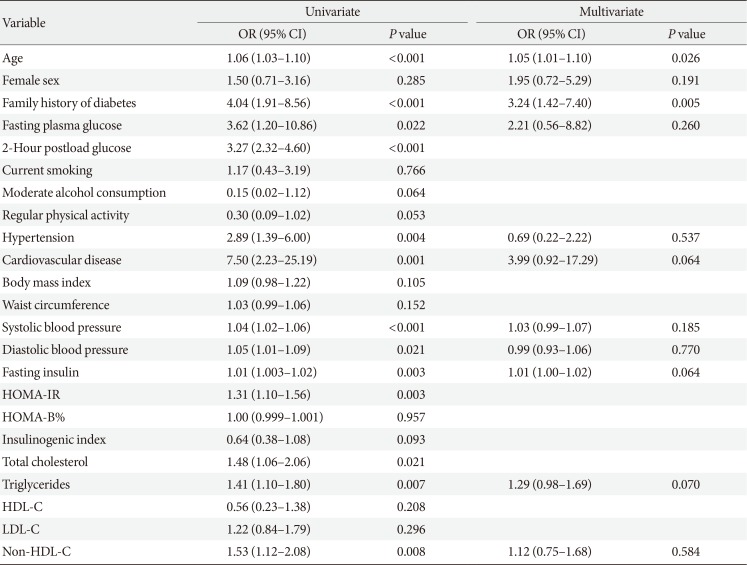




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download